The Essential Guide to Requirements Management and Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- Glossary
Chapter 6: Best Practices for Verification and Validation in Product Development
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- Glossary
Best Practices for Verification and Validation in Product Development
In the competitive landscape of modern product development, ensuring the reliability and quality of the product is essential to meet customer – and stakeholder – expectations and regulatory requirements. Verification and validation (V&V) are two crucial processes that play a pivotal role in achieving these goals. V&V are systematic methods that assess a product’s adherence to specifications and its ability to perform as intended. In this article, we will delve into the best practices for verification and validation in product development, exploring the key steps, methodologies, and benefits of each process.
Understanding Verification & Validation:
Before delving into the best practices, it is essential to clarify the distinction between verification and validation. Verification focuses on assessing whether a product meets its design specifications, ensuring that each component and feature works as intended. On the other hand, validation is concerned with evaluating whether the product fulfills its intended use and customer needs. In essence, verification confirms if the product is designed correctly, while validation confirms if it is the right product for the intended application.
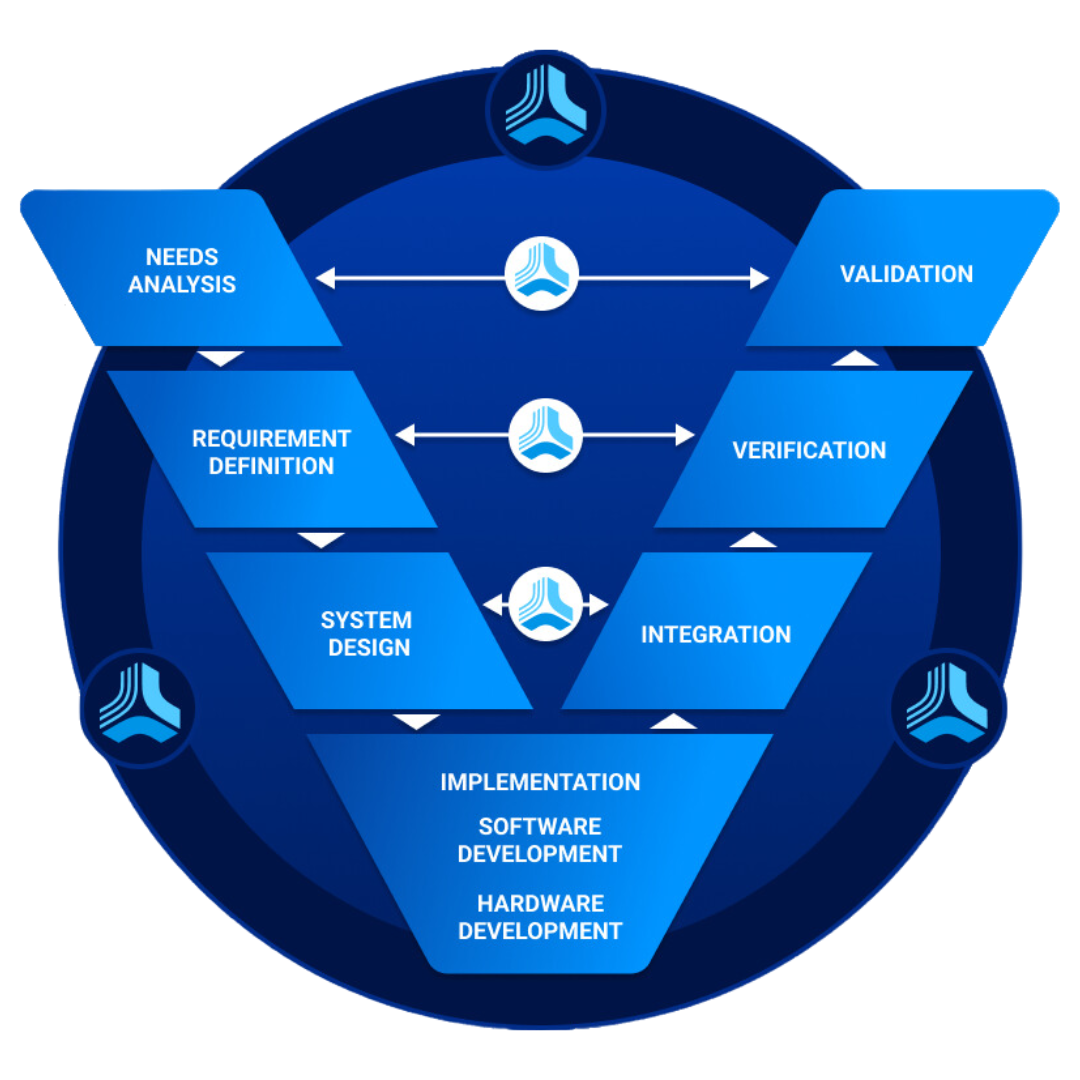
Incorporating V&V Early in the Development Lifecycle:
To maximize the effectiveness of verification and validation, these processes must be integrated into the product development lifecycle from its early stages. By starting V&V activities early, potential issues can be identified and resolved before they escalate, reducing costs and time-to-market. Early involvement also allows for feedback to be incorporated into the design, leading to a more robust and reliable final product.
Clearly Defined Requirements:
Well-defined requirements are the foundation of successful verification and validation. During the requirements gathering phase, it is vital to engage stakeholders and subject matter experts to create clear, measurable, and unambiguous specifications. These requirements serve as the baseline against which the product will be verified and validated. Proper documentation and version control are critical to ensure that changes to requirements are tracked effectively. Additionally, the later in the development process that requirements get changed, many times because they weren’t written well the first time, the more costly it is due to downstream impacts such as rework in verification and validation.
RELATED ARTICLE: Plutora – Verification vs Validation: Do You Know the Difference?
Utilizing Various V&V Techniques:
Product development teams should employ a mix of V&V techniques to comprehensively assess the product’s quality. Some commonly used methods include:
- Testing: Conduct thorough testing, including unit testing, integration testing, system testing, and user acceptance testing, to verify that each component and the product as a whole performs as expected.
- Simulation: Use computer simulations to evaluate the product’s behavior in various scenarios, particularly for complex systems or when physical testing is impractical or cost prohibitive.
- Prototyping: Building prototypes early in the development process allows for real-world testing, uncovering potential design flaws and usability issues.
- Peer Reviews: Encourage regular peer reviews of design documents, code, and other artifacts to catch errors and improve the overall quality of the product.
- Model-based Design: Utilize model-based design approaches, such as Model-Driven Architecture (MDA), to create detailed models that can be verified before implementation.
Risk-Based Approach:
Incorporate a risk-based approach into V&V activities to focus resources on critical areas. Identify potential risks associated with product failure and prioritize verification and validation efforts accordingly. This approach ensures that resources are allocated efficiently, concentrating on areas with the most significant impact on product performance and safety.
Independent Verification and Validation (IV&V):
Consider engaging external experts or teams for independent verification and validation. External parties can provide an unbiased assessment of the product, uncovering issues that internal teams might overlook due to familiarity or assumptions. Independent verification and validation bring additional expertise and ensure a higher level of confidence in the product’s quality.
Continuous Integration and Continuous Delivery (CI/CD):
Implementing CI/CD practices allows for continuous verification and validation throughout the development process. Automated testing and deployment pipelines can quickly detect regressions and integration issues, ensuring that the product remains stable and reliable throughout its evolution.
Documenting V&V Activities:
Comprehensive documentation of all verification and validation activities is essential for compliance, knowledge retention, and continuous improvement. Properly documented V&V processes help maintain a historical record of changes, failures, and resolutions, facilitating future product iterations and troubleshooting.
V & V are integral to successful product development, ensuring that products meet the required specifications and perform as intended. By adopting best practices such as early integration, clear requirements, a mix of v&v techniques, risk-based approaches, and continuous verification, companies can create high-quality, reliable products that customers love and gain a competitive edge in the market. Moreover, investing in verification and validation from the outset of development can save time and resources, prevent costly delays, and lead to higher customer satisfaction and loyalty in the long run.
In This Webinar, We Discuss How to Write Better Requirements with Jama Connect Advisor™
Verification and Validation (V&V) are independent procedures that are used together for checking that a product, service, or system meets requirements and specifications and that it fulfills its intended purpose. These are critical components of a quality management system such as ISO 9000. The words “verification” and “validation” are sometimes preceded with “independent”, indicating that the verification and validation is to be performed by a disinterested third party. “Independent verification and validation” can be abbreviated as “IV&V”.
Book a Demo
See Jama Connect in Action!
Our Jama Connect experts are ready to guide you through a personalized demo, answer your questions, and show you how Jama Connect can help you identify risks, improve cross-team collaboration, and drive faster time to market.