What is Traceability?
The Essential Guide to Requirements Management and Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
Chapter 4: What is Traceability?
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
What is Traceability?
The Definition of Requirements Traceability
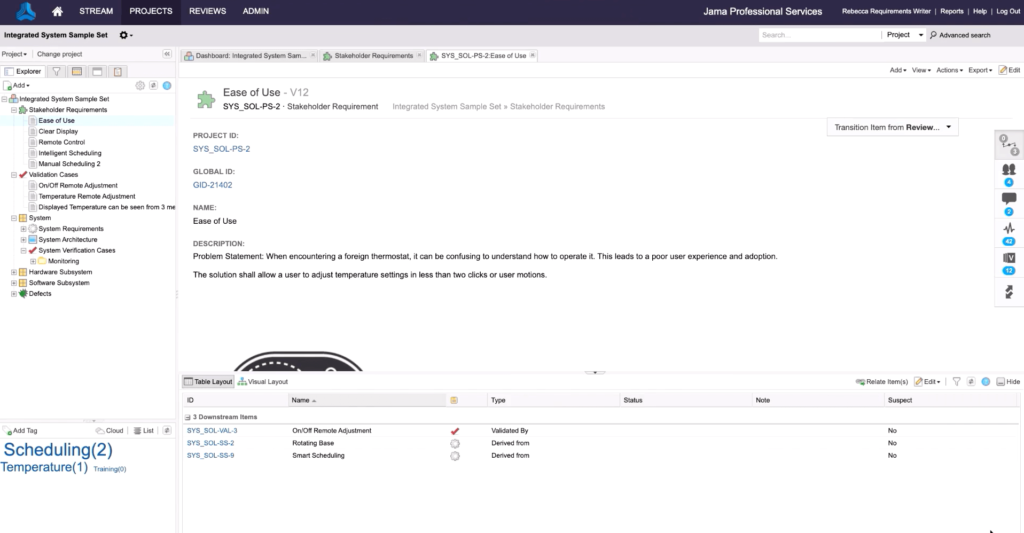
Requirements traceability is the ability to track and document the lineage and history of requirements throughout the development process. It provides forward and backward visibility into all activities surrounding each requirement, including design, development, testing, and support, by keeping track of components, parts, materials, and processes involved in the product’s creation. This documented thread helps minimize the risk of negative outcomes, maximize productivity, and ensure that products meet required specifications and standards. Benefits include greater team efficiency, easier regulatory compliance, and higher-quality products, and it allows for quick identification and resolution of any defects or problems.
RELATED ARTICLE: Write Better Requirements with Jama Connect Advisor™
What is the Purpose and Importance of Traceability?
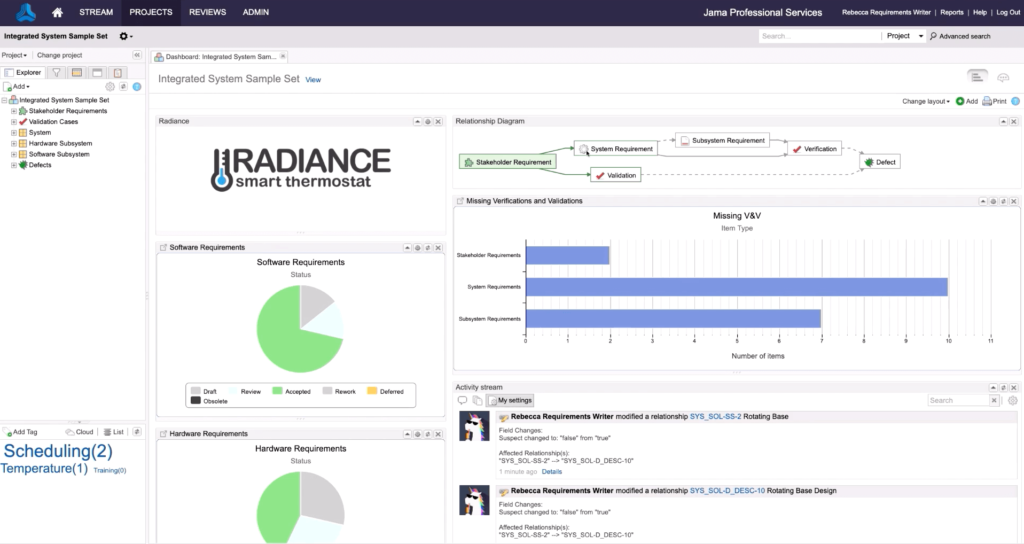
Traceability enables product teams to associate a specific requirement with all the related project artifacts, as well as other requirements, both forward and backward, so that anyone can see how the activity relates to the requirement — and vice versa — at any point during development. This functionality, also called live traceability, fosters team collaboration and enables early detection of possible production risks.
Think of it this way: How important is it to have your products developed correctly in terms of definition, quality, and timing? Mission critical, right? That’s why requirements traceability is so critical.
Traceability is particularly important in industries such as aerospace, automotive, and medical devices, where safety, quality, and compliance are critical. In these industries, traceability systems are often mandated by regulatory bodies to ensure that products are safe, reliable, and comply with applicable standards and regulations.
Let’s take a look at some of the benefits of requirements traceability throughout the development process. For example:
- Simplifies project estimates
- Enhances process visibility
- Increases development efficiency
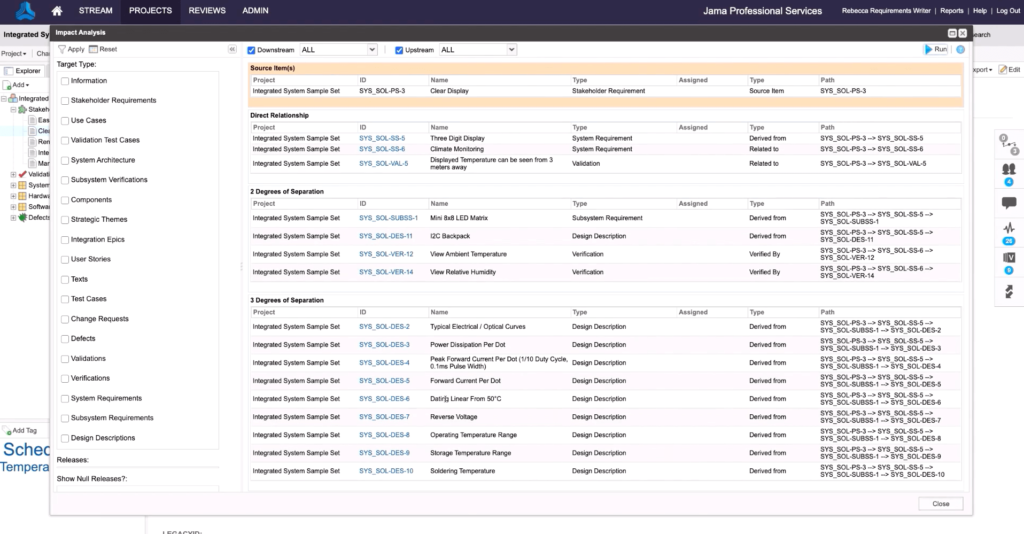
- Improves impact analysis of change
- Demonstrates verification and validation
- Strengthens product quality
- Proves compliance or functional safety
A single line of sight on a requirement, or a digital thread, is supremely important for application lifecycle management (ALM) and product lifecycle management (PLM). Both endure tight timelines and increasing numbers of requirements — including those from regulations, where compliance is non-negotiable.
Traceability is particularly important in industries such as aerospace, automotive, and medical devices, where safety, quality, and compliance are critical. In these industries, traceable systems are often mandated by regulatory bodies to ensure that products are safe, reliable, and comply with applicable standards and regulations.
Knowing the relationship between requirements, risk, tests, and so forth, is the difference between developing a compliant product on time and getting stuck in rework, delaying launches, and dealing with unhappy stakeholders. With requirements traceability, you don’t have to compromise on speed or quality.
The bottom line is that end-to-end traceability confirms you’re building the right thing and helps you prove compliance or functional safety. Without it, your development efficiency and product quality are in jeopardy.
What is a Traceable System?
A traceable product or system is one that has a documented history or record of its development, production, and testing. This record provides a complete picture of the product or system, including its design, components, and any modifications or changes made throughout its development. A traceable system also enables organizations to quickly identify the source of any issues or problems that arise during production or testing, and to take corrective action as needed.
RELATED ARTICLE: Requirements Traceability Benchmark
Forward, backward, and bidirectional: The mechanics of creating a digital thread with traceability
To connect all the phases of the development process, from customer needs through support, four distinct kinds of links must be used to thread the process end-to-end. This includes high-level requirements as well as derived requirements — those not specifically defined but necessary for meeting defined requirements or having the system work as expected. Derived requirements must also be adequately traced to reap the full benefits of Live Traceability™.
Forward
There are two kinds of forward traceability: forward to requirements and forward from requirements. They both trace from an upstream component to downstream artifacts. The difference is in where they begin.
Forward to requirements traces from customer need to requirements. This is important because customer needs can evolve over time. If they do, requirements may need to change as a result. Following this type of forward traceability enables teams to be informed of changes in priorities at any time throughout development.
Forward from requirements traces relationships between requirements and corresponding downstream artifacts, including test cases. This type of trace ensures that each requirement is not only satisfied but verified and validated.
Backward
Like forward, there are two kinds of backward traceability. This pair traces from an endpoint, or downstream work product, to upstream elements. These two types have differing starting points.
Backward from requirements gives insight on how a requirement came to be by linking the requirement to the customer use case it addresses. This enables teams to improve decision making by understanding the origin of a requirement.
Backward to requirements begins at performed work and traces to its requirement. It gives visibility into why specific items were created and how different pieces of a system fit together. Tracing in this way also allows testers to find gaps or missing requirements.
Bidirectional
Bidirectional requirements traceability is the ability to perform both forward and backward traceability. Bidirectional is optimal because it gives teams full visibility from requirements specifications through building, testing, changes, defects, and back again. Traceability of this caliber can only be achieved through automated requirements management tools, such as Jama Connect®.
What Are the Benefits of Traceability?
The benefits of traceability are many. First, it ensures product quality by enabling organizations to track and verify that all components and processes meet the required standards and specifications. This helps to reduce the risk of defects and recalls, which can be costly in terms of both financial losses and damage to a company’s reputation.
Second, traceability helps to ensure compliance with regulatory requirements. In many industries, organizations are required to maintain detailed records of their products and processes and to demonstrate that they are meeting all applicable standards and regulations. A traceable system provides a way to easily produce these records and demonstrate compliance.
Third, it promotes accountability and transparency. By maintaining a complete record of a product’s development and testing, organizations can demonstrate that they are taking steps to ensure quality and safety. This can help to build trust with customers and stakeholders and enhance a company’s reputation.
How Do You Enable Traceability?
In practice, traceability involves the use of tools and systems to track and document the various components and processes involved in product development and testing. These tools can range from simple spreadsheets and databases to modern requirements management platforms that generate Live Traceability™ that integrate with other product development tools such as product lifecycle management (PLM) systems, test management tools, and task management tools like Atlassian Jira and Azure DevOps.
Challenges in Implementing Traceability
One of the key challenges in implementing traceability is ensuring that all relevant data is captured and documented. This can be particularly difficult in complex product development processes involving multiple teams, suppliers, and partners. However, by establishing clear processes and standards for data capture and documentation, organizations can overcome these challenges and reap the benefits of traceability.
In conclusion, traceability is a critical aspect of product and system development that enables organizations to ensure product quality, compliance, and accountability. By maintaining a complete record of a product’s development and testing, organizations can reduce the risk of defects and recalls, demonstrate compliance with regulatory requirements, and build trust with customers and stakeholders. While implementing a traceability system manually is very challenging, using a modern platform like Jama Connect automatically creates Live Traceability throughout the development process.
Note: This article was partially drafted with the aid of AI. Additional content, edits for accuracy, and industry expertise by McKenzie Jonsson, Decoteau Wilkerson, and Karrie Sundbom.
The Importance of Requirements Traceability in Complex Projects
Requirements traceability is a critical practice in managing complex projects across industries, ensuring every requirement is clearly connected to its origins, implementation, and verification. This “trace-ability” enhances project visibility, streamlines collaboration, and simplifies compliance with industry standards. But what exactly does “traceable” mean?
To define trackable, it involves the ability to monitor and verify requirements through every stage of the development lifecycle. In other words, traceability ensures that requirements are not just documented but also consistently “traced” to corresponding deliverables, including design elements, test cases, and risk assessments. The traced meaning goes beyond simple documentation—it’s about creating a live, dynamic connection between project artifacts.
What is Compliance Traceability?
For organizations operating in regulated industries, compliance traceability is vital for adhering to standards such as ISO 26262, DO-178C, FDA Design Controls, and others. By leveraging requirements traceability software, teams can ensure that all requirements meet regulatory mandates, reducing the risk of costly non-compliance or project delays.
Modern traceability tools provide powerful capabilities to define, track, and manage these connections. These tools make it easier to answer critical questions during audits, such as:
- Which requirement is linked to a specific test case?
- What is the status of risk assessments associated with each requirement?
- How do design changes impact downstream deliverables?
Why Use Requirements Traceability Software?
The evolution of requirement tracing tools has redefined how teams manage complex projects. Modern software enables teams to achieve live traceability, where all relationships between requirements and project elements are automatically updated as changes occur. With the right software tracing tools, teams can save time, minimize errors, and improve collaboration.
Key benefits of requirements traceability software include:
- Real-time insights into project progress and risk.
- Simplified compliance reporting with audit-ready documentation.
- Streamlined workflows that connect cross-functional teams.
Traceable Definition in Modern Requirements Management
When you trace define requirements in a project, you’re establishing a roadmap to connect all related elements—from initial stakeholder needs to final deliverables. A traceable definition incorporates several elements:
- Identifiable: Each requirement must be uniquely defined and accessible.
- Linked: Requirements must be “traced” to other relevant artifacts, such as test cases or risks.
- Verified: The trace must be validated through testing and other means to ensure accuracy.
In this context, traceability tools are indispensable for meeting project goals and maintaining quality.
The Role of Traceability in Driving Innovation
In today’s competitive landscape, effective traceability isn’t just a compliance measure—it’s a strategic advantage. When teams use the best requirement tracing tools, they can better manage complexity, identify potential risks early, and deliver higher-quality products faster.
Understanding and implementing traceability practices with advanced tools can transform how your organization operates, setting the stage for greater innovation, efficiency, and success.
Editors note: This article was partially drafted with the assistance of AI and reviewed for accuracy by McKenzie Jonsson.
In This Webinar, Learn How Jama Connect® Helps Maintain Live Traceability™ Across Applications.
Requirements Traceability: is the tracking of requirements throughout the product development lifecycle.
Book a Demo
See Jama Connect in Action!
Our Jama Connect experts are ready to guide you through a personalized demo, answer your questions, and show you how Jama Connect can help you identify risks, improve cross-team collaboration, and drive faster time to market.