Live Traceability vs. After-the-Fact Traceability
The Essential Guide to Requirements Management and Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- Glossary
Chapter 4: Live Traceability vs. After-the-Fact Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- Glossary
Live Traceability vs. After-the-Fact Traceability
In today’s world, successful organizations anchor their innovation and complex product development processes on interconnected data across numerous workstreams. This requires gathering stakeholder input to build system architecture, managing high-level requirements to create detailed user stories and implementing verification and validation to detect issues. Collaborating with various stakeholders while achieving standards compliance and competing in today’s marketplace requires a deeper level of traceability.
Requirements traceability is required by many industry standards to ensure product quality and safety. The industry standards are based on decades of progress made in systems and quality engineering research with requirements traceability at the core. Benefits from requirements traceability are achieved if and only if traceability is used as a tool during the product development process.
These benefits include greatly reduced or eliminated delays, defects, cost overruns, and rework. Here is an overview of the best practice approach to achieve Live Traceability™.
Live Traceability vs. After-the-fact Traceability
Let’s start with some definitions to make sure we are all on the same page. Requirement traceability is defined as tracking the development progress of product requirements from definition and design through development, testing, verification, and validation. There are two forms of requirement traceability: after-the-fact traceability and Live Traceability.
RELATED ARTICLE: Experience Live Traceability™
What is traditional, after-the-fact Traceability?
After-the-fact traceability occurs after the product has been developed and is typically a highly manual effort to try and re-create artifacts to demonstrate traceability that should have occurred during the development process but did not. This effort is undertaken solely to comply with industry standards and satisfy auditor requests for demonstration of process maturity.
What is Live Traceability?
Jama Software defines Live Traceability ™ as the ability to see the most up-to-date and complete upstream and downstream information for any requirement, no matter the stage of systems development or how many siloed tools and teams it spans.
This enables engineering and product management processes to be managed through data and to improve performance in real-time.
Live Traceability occurs in real time as the product development process progresses to improve overall productivity (by ensuring engineers across disciplines are always working off the most recent and correct versions) and to reduce the risk of negative product outcomes (delays, defects, rework, cost overruns, recalls, etc.) through early detection of issues.
The benefits of early detection of issues are significant. Research by INCOSE found that issues not found until verification and validation are 40 to 110 times more costly than if found during design. For this reason, most companies want Live Traceability but are stuck with legacy tools and spreadsheets that do not support it. Since each engineering discipline is allowed to choose its own tooling, the result is a large number of tools with no relationship rules or mechanisms to create Live Traceability across them.
Why does Live Traceability matter?
The #1 problem product engineering organizations face is complying with traceability requirements spanning siloed teams and tools. Organizations are often encumbered by the highly manual and time-consuming review of information across numerous spreadsheets.
Live Traceability™ Realized
Achieve Live Traceability across your best-of-breed toolchain. To make this continuous sync as easy as possible, Jama Connect Interchange is purpose-built to achieve Live Traceability between Jama Connect and Jira through a point and click interface.
Key Benefits of Integrating Jama Connect with Jira for Live Traceability Stay Aligned to Market and User Needs
Integrate upstream planning, requirements, and test plans into an iterative development process. Ensure what you build satisfies market, compliance, and user requirements.
Maintain Visibility into Downstream Development
Accurately capture and communicate requirements, goals, progress, and interdependencies to remove friction throughout the development process.
Eliminate Late-Stage Rework and Quality Gaps Due to Misalignment
Capture needs and maintain agreement on what you are building. Guarantee everyone is working off the most current spec, so the product/system ultimately delivers its intended value.
Support a Formal Change Management Process
Identify change impact implications of product requirements alterations and impacted owners so development teams make informed decisions as requirements evolve.
So how do you achieve Live Traceability?
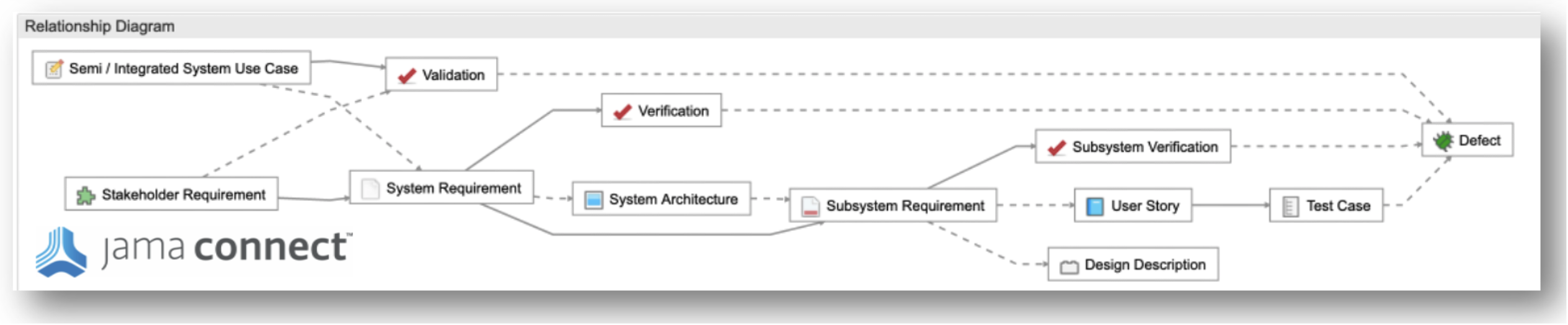
Step 1: Define a Traceability Model
Live Traceability requires a model of the key process elements and their relationship rules to monitor during the development process. The systems engineering V Model is a useful framework to start with for data object and relationship definition. Jama Connect® uniquely provides a point and click, configurable, relationship rule capability to enable Live Traceability. Below you see a sample relationship rule diagram from Jama Connect. Relationship rules vary by industry and company-specific requirements. Best practice templates are provided to comply with industry standards and configured to meet client-specific needs. The definition of a traceability model forms the foundation for model-based systems engineering since it defines model elements and their relationship to each other in a consistent manner across the entire system architecture.
Step 2: Setup Continuous Sync for Siloed Tools/Spreadsheets
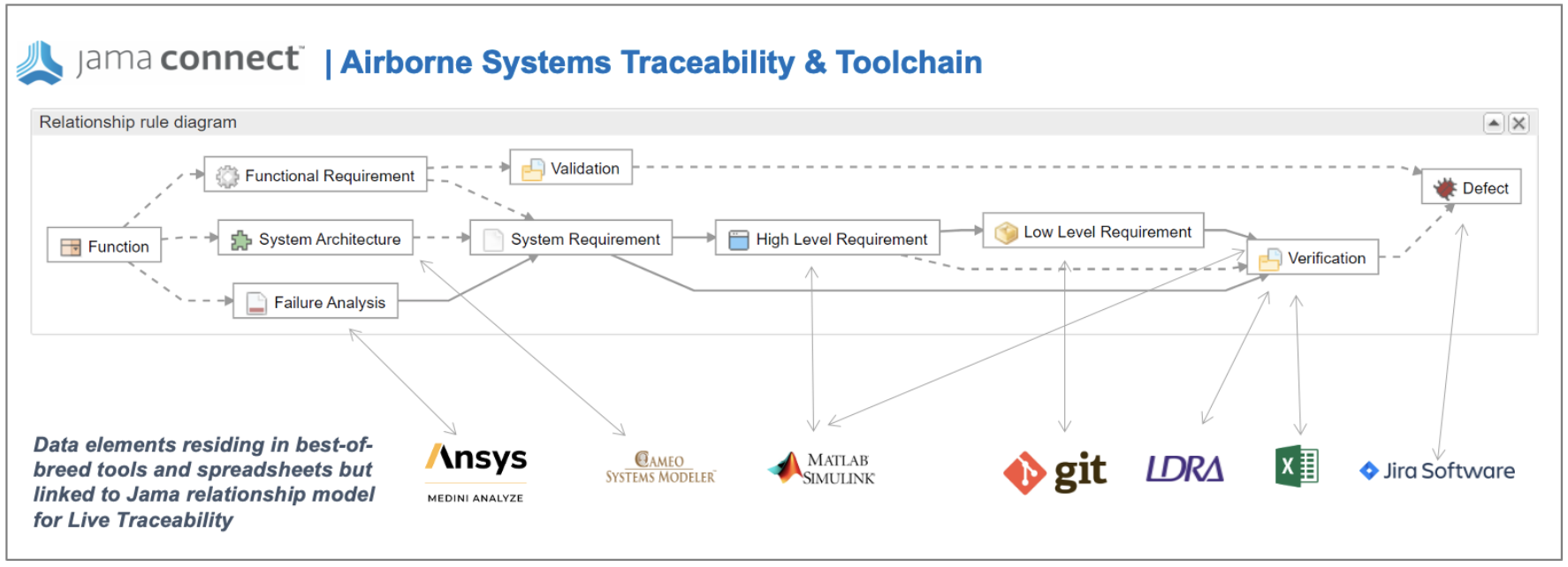
Once the relationship rules are defined, the next step is to set up continuous sync with best-of-breed tools and spreadsheets used by the various engineering disciplines. The traceability diagram below shows a typical example of best-of-breed tools and where they sync in the Jama Connect relationship model to deliver Live Traceability.
Critical, best-of-breed tools for traceability are shown in this example. Additional and competitive alternatives to those listed are also supported.
Most companies prioritize the areas of the traceability model that are most prone to lead to costly issues in the absence of a continuous sync. Most commonly, these areas are:
- Software task management – directly linking the decomposition of requirements into user stories enables Live Traceability through the software development process through testing and defect management. The most common best-of-breed tools used are Jira and Azure Dev Ops.
- Test automation – test cases are managed in Jama Connect to align to requirements and ensure traceability across all engineering disciplines with the test automation results sync’d to the traceability model at the verification step. The most common test automation tools are TestRail and qTest.
- Risk analysis (DFMEA/FMEA) – is most often conducted in multiple Microsoft Excel spreadsheets and the assumption has been that Live Traceability was not possible with Excel. Jama Connect is the first requirements management solution to enable Live Traceability with Excel functions and spreadsheets. Risk teams can now work in their preferred spreadsheets AND for the first time achieve live traceability to stay in sync with changes made by any engineering team. Ansys Medini is also a supported integration.
- Model-based systems engineering (MBSE) – the first step in MBSE is to define a relationship model between all product requirements. Once a relationship model is defined, then specifications can be determined through modeling. Jama Connect uniquely provides model-based requirements to sync logically with a SysML modeling tool like Cameo No Magic. Other requirements management tools do not ensure a model-based approach, which most often leads to inconsistent and conflicting fields across teams and projects and provides no coherent relationship model.
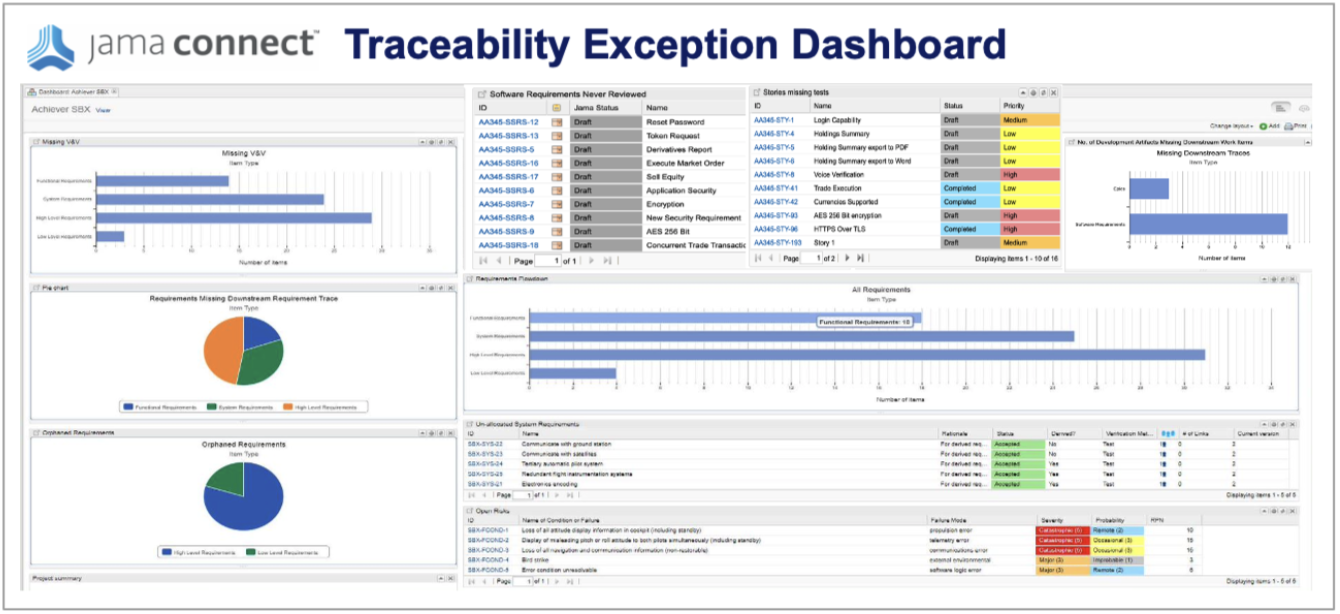
Step 3: Monitor for Exceptions
Live Traceability provides the ability, for the first time, to manage by exception the end-to-end product development process across all engineering disciplines. The traceability model defines expected process behavior that can be compared to actual activity to generate exceptions. These exceptions are the early warning indicators of issues that most often lead to delays, cost overruns, rework, defects, and recalls. Below is a sample exception management dashboard in Jama Connect.
Benefits of Live Traceability
The main benefits of Live Traceability across best-of-breed tools are as follows:
- Reduce the risk of delays, cost overruns, rework, defects, and recalls with early detection of issues through exception management and save 40 to 110 times the cost of issues identified late in the process.
- Comply with industry standards with no after-the-fact manual effort.
- No disruption to engineering teams that continue working in their chosen best-of-breed tools with no need to change tools, fields, values or processes.
- Increase productivity and satisfaction of engineers with the confidence that they are always working on the latest version, reflective of all changes and comments.
In This Webinar, We Cover Best Practices for Requirements Traceability
Live Traceability is the ability to see the most up-to-date and complete upstream and downstream information for any requirement, no matter the stage of systems development or how many siloed tools and teams it spans.
Book a Demo
See Jama Connect in Action!
Our Jama Connect experts are ready to guide you through a personalized demo, answer your questions, and show you how Jama Connect can help you identify risks, improve cross-team collaboration, and drive faster time to market.