TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
The Essential Guide to Requirements Management and Traceability
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
Chapter 7: TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
Chapters
- 1. Requirements Management
- Overview
- 1 What is Requirements Management?
- 2 Why do you need Requirements Management?
- 3 Four Fundamentals of Requirements Management
- 4 Adopting an Agile Approach to Requirements Management
- 5 Status Request Changes
- 6 Conquering the 5 Biggest Challenges of Requirements Management
- 7 Three Reasons You Need a Requirements Management Solution
- 2. Writing Requirements
- Overview
- 1 Functional requirements examples and templates
- 2 Identifying and Measuring Requirements Quality
- 3 How to write system requirement specification (SRS) documents
- 4 The Fundamentals of Business Requirements: Examples of Business Requirements and the Importance of Excellence
- 5 Adopting the EARS Notation to Improve Requirements Engineering
- 6 Jama Connect Advisor™
- 7 Frequently Asked Questions about the EARS Notation and Jama Connect Advisor™
- 8 How to Write an Effective Product Requirements Document (PRD)
- 9 Functional vs. Non-Functional Requirements
- 10 What Are Nonfunctional Requirements and How Do They Impact Product Development?
- 11 Characteristics of Effective Software Requirements and Software Requirements Specifications (SRS)
- 12 8 Do’s and Don’ts for Writing Requirements
- 3. Requirements Gathering and Management Processes
- Overview
- 1 Requirements Engineering
- 2 Requirements Analysis
- 3 A Guide to Requirements Elicitation for Product Teams
- 4 Requirements Gathering Techniques for Agile Product Teams
- 5 What is Requirements Gathering?
- 6 Defining and Implementing a Requirements Baseline
- 7 Managing Project Scope — Why It Matters and Best Practices
- 8 How Long Do Requirements Take?
- 4. Requirements Traceability
- Overview
- 1 What is Traceability?
- 2 Tracing Your Way to Success: The Crucial Role of Traceability in Modern Product and Systems Development
- 3 Change Impact Analysis (CIA): A Short Guide for Effective Implementation
- 4 What is Requirements Traceability and Why Does It Matter for Product Teams?
- 5 Key Traceability Challenges and Tips for Ensuring Accountability and Efficiency
- 6 Unraveling the Digital Thread: Enhancing Connectivity and Efficiency
- 7 The Role of a Data Thread in Product and Software Development
- 8 How to Create and Use a Requirements Traceability Matrix
- 9 Traceability Matrix 101: Why It’s Not the Ultimate Solution for Managing Requirements
- 10 Live Traceability vs. After-the-Fact Traceability
- 11 How to Overcome Organizational Barriers to Live Requirements Traceability
- 12 Requirements Traceability, What Are You Missing?
- 13 Four Best Practices for Requirements Traceability
- 14 Requirements Traceability: Links in the Chain
- 15 What Are the Benefits of End-to-End Traceability During Product Development?
- 5. Requirements Management Tools and Software
- Overview
- 1 Selecting the Right Requirements Management Tools and Software
- 2 Why Investing in Requirements Management Software Makes Business Sense During an Economic Downturn
- 3 Why Word and Excel Alone is Not Enough for Product, Software, and Systems Development
- 4 Application lifecycle management (ALM)
- 5 Is There Life After DOORS®?
- 6 Checklist: Selecting a Requirements Management Tool
- 6. Requirements Validation and Verification
- 7. Meeting Regulatory Compliance and Industry Standards
- Overview
- 1 Understanding ISO Standards
- 2 Understanding ISO/IEC 27001: A Guide to Information Security Management
- 3 What is DevSecOps? A Guide to Building Secure Software
- 4 Compliance Management
- 5 What is FMEA? Failure Modes and Effects Analysis
- 6 TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
- 8. Systems Engineering
- 9. Automotive Development
- 10. Medical Device & Life Sciences Development
- Overview
- 1 The Importance of Benefit-Risk Analysis in Medical Device Development
- 2 Software as a Medical Device: Revolutionizing Healthcare
- 3 What’s a Design History File, and How Are DHFs Used by Product Teams?
- 4 Navigating the Risks of Software of Unknown Pedigree (SOUP) in the Medical Device & Life Sciences Industry
- 5 What is ISO 13485? Your Comprehensive Guide to Compliant Medical Device Manufacturing
- 6 What You Need to Know: ANSI/AAMI SW96:2023 — Medical Device Security
- 7 ISO 13485 vs ISO 9001: Understanding the Differences and Synergies
- 8 Failure Modes, Effects, and Diagnostic Analysis (FMEDA) for Medical Devices: What You Need to Know
- 9 Embracing the Future of Healthcare: Exploring the Internet of Medical Things (IoMT)
- 11. Aerospace & Defense Development
- 12. Architecture, Engineering, and Construction (AEC industry) Development
- 13. Industrial Manufacturing & Machinery, Automation & Robotics, Consumer Electronics, and Energy
- 14. Semiconductor Development
- 15. AI in Product Development
- Glossary
TÜV SÜD: Ensuring Safety, Quality, and Sustainability Worldwide
In an ever-evolving world where safety, quality, and sustainability are paramount concerns, TÜV SÜD stands as a trusted global leader in providing expert services that guarantee these vital elements across various industries. With a rich history dating back over a century, TÜV SÜD has become synonymous with reliability and excellence, offering a wide range of services that help organizations meet and exceed international standards.
A Legacy of Excellence
Founded in Germany in 1866, TÜV SÜD has a long and storied history of commitment to ensuring the highest levels of safety and quality. The organization’s name, TÜV, is an abbreviation for “Technischer Überwachungsverein,” which translates to “Technical Inspection Association.” This name reflects the organization’s original purpose: inspecting and certifying the safety of steam boilers. Over the years, TÜV SÜD has expanded its scope to encompass a multitude of industries, making it a globally recognized name in testing, inspection, and certification.
Comprehensive Services
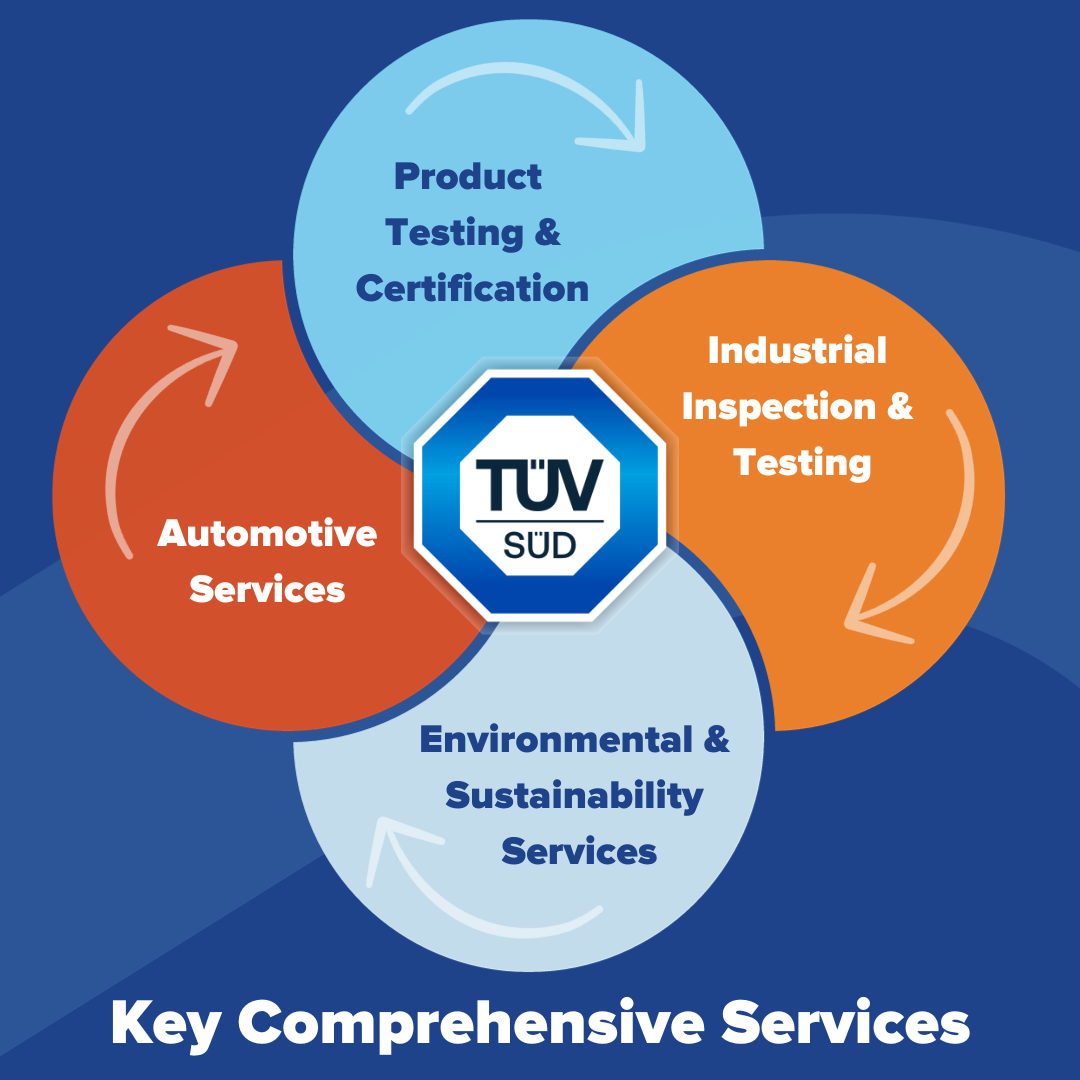
TÜV SÜD’s portfolio of services is extensive, covering a wide array of sectors such as automotive, healthcare, manufacturing, energy, and more. Here are some of the key services offered by TÜV SÜD:
**1. Product Testing and Certification:
TÜV SÜD tests and certifies products to ensure they meet international safety and quality standards. Whether it’s electronics, machinery, or consumer goods, their certifications provide a mark of quality and safety that instills confidence in both consumers and businesses.
**2. Industrial Inspection and Testing:
The organization conducts rigorous inspections and testing for industrial equipment, facilities, and processes. This ensures that businesses can operate safely and efficiently while complying with relevant regulations.
**3. Environmental and Sustainability Services:
With a growing emphasis on sustainability, TÜV SÜD offers services to help organizations reduce their environmental impact. From energy efficiency assessments to carbon footprint evaluations, TÜV SÜD aids businesses in their journey towards a greener future.
**4. Automotive Services:
TÜV SÜD provides comprehensive automotive testing and certification services, ensuring vehicles and automotive components meet stringent safety and quality standards.
Global Reach and Expertise
TÜV SÜD operates in more than 1,000 locations across the globe, with a team of over 26,000 experts. This vast network allows the organization to provide localized services with a global perspective. Their experts possess a deep understanding of industry-specific challenges, enabling them to offer tailored solutions that address clients’ unique needs.
RELATED ARTICLE: Compliance Management
Driving Innovation and Safety Forward

One of TÜV SÜD’s core missions is to promote innovation while maintaining safety and quality. They work closely with businesses to help them navigate complex regulatory landscapes and develop cutting-edge technologies that comply with international standards. This approach fosters innovation while ensuring that products and processes meet the highest safety and quality benchmarks.
The Future of Assurance
As industries continue to evolve, TÜV SÜD remains at the forefront of providing assurance and certification services. Their commitment to safety, quality, and sustainability ensures that businesses can thrive in an increasingly competitive and complex world. By choosing TÜV SÜD, organizations gain a trusted partner dedicated to helping them achieve excellence and compliance in their respective fields.
RELATED ARTICLE: What is DevSecOps? A Guide to Building Secure Software
Conclusion
In a world where trust, safety, and quality are paramount, TÜV SÜD shines as a beacon of assurance. With a rich history, a global presence, and a commitment to excellence, TÜV SÜD continues to play a vital role in ensuring the success and safety of businesses across various industries. For those seeking partners in quality, innovation, and sustainability, TÜV SÜD stands as a trusted ally, driving the future of assurance forward.
To learn more about how Jama Connect can help your team simplify compliance, streamline development, and speed time to market, download our solution overview. >> https://resources.jamasoftware.com/datasheet/jama-connect
In This Webinar, See Firsthand How Jama Connect® Helps Teams Reduce Unacceptable Risks Through Compliance Management
TÜV SÜD: TÜV, is an abbreviation for “Technischer Überwachungsverein,” which translates to “Technical Inspection Association.”
Book a Demo
See Jama Connect in Action!
Our Jama Connect experts are ready to guide you through a personalized demo, answer your questions, and show you how Jama Connect can help you identify risks, improve cross-team collaboration, and drive faster time to market.