Traceability is the tracking of requirements across the product development cycle. It documents the status of everything being worked on and shows the history of development along with the impacts of specific changes. Its benefits include easier regulatory compliance, more in-sync teams, and higher-quality releases.
A dedicated software traceability solution may be relied upon to systematically track and trace a requirement’s life. Compared to using discrete documents (e.g., spreadsheets) for the same purpose, this type of centralized platform lets teams make easier, more accurate assessments that support more informed decisions about products. It can dependably identify who made each change, what the change entailed, and why it was made – all in one system of record.
Accordingly, such a platform enables superior software requirements testing and management, in industries as varied as medical device manufacturing and automotive production. Let’s look at five tips for how to improve requirements traceability, along with how traceability software helps with each one.
1. Use Real-Time Communications To Empower Teams
Many requirements traceability processes are highly manual and disconnected:
- For example, Team Member A creates a traceability matrix in Microsoft Excel. This matrix is a table containing artifacts like requirements, test cases, test runs, and identified issues. Its purpose is to show that compliance requirements for a project are being met.
- However, the artifacts in it get frequently updated during the course of development and testing. So Team Member A goes back in periodically to update the traceability matrix and make sure it is fully up-to-date. This is time-consuming and error-prone work.
- Team Member B then has to dig through this matrix to see if the changes are relevant to them. It’s possible that what they see at any given point isn’t accurate, though. Keeping up with changes is its own job, with many back-and-forth email barrages to navigate.
Real-time communications within a traceability solution alleviate these issues. Instead of hopping between static documents scattered across emails, team members can collaborate in one virtual space, in which they can share feedback, participate in livestream discussions, see connections between items and their authors or editors, and expedite reviews and approvals. They also have access to all comments, test cases, and activity streams from the same interface.
Such features ensure that changes are communicated directly to the relevant parties and that software traceability stays on track. Team members do not have to waste time poring over a document that might not be 100% accurate or even applicable to their particular responsibilities. Basically, real-time communications remove the traditional bottlenecks of hunting for the latest updates and wondering about their relevance.
2. Chart the Impact of a Change Before it Happens
As noted above, requirements change all the time during software development. It is essential not only to keep team members in sync about these changes, but also to scope out their full impact across the product development lifecycle.
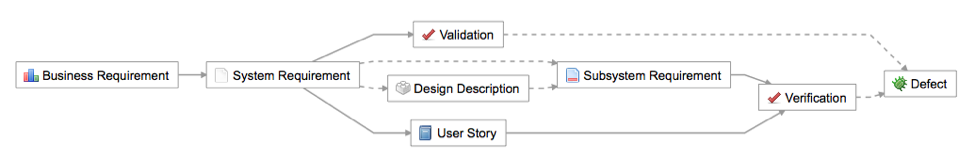
Altering one requirement will directly affect any related system requirements, along with downstream requirements and numerous verification tests. Teams need insight into what these changes will entail, so that they can see if related items are still correct, make any necessary updates, and ultimately trace the right requirements for quality and compliance purposes.
Live traceability in Jama Connect allows for better impact analysis and more streamlined navigation of upstream and downstream relationships:
- For instance, links downstream from modified items are automatically flagged as “suspect” to alert team members to the need for possible action.
- Relevant contributors can also be notified right away, and critical decisions prioritized; meanwhile, everyone else can save time by not being pulled into something irrelevant.
- System users can see if a requirement has test cases downstream and what percentage of them have passed.
- With all risks and requirements being updated in real time, teams can reliably trace them and conduct informed evaluations and analyses.
Through these capabilities, a traceability solution helps everyone be more confident that they are working with the right requirements, while avoiding getting bogged down in rework or in costly late-stage changes caused by not having an accurate picture of what is being worked on at every stage. In other words: Live traceability means that traceability is no longer an afterthought.
3. Create a Detailed Audit Trail With Well-Documented Changes
One of the main reasons for implementing traceability software is to simplify regulatory compliance. Let’s say that a hypothetical medical device manufacturer is planning to bring a new connected device to the U.S. market.
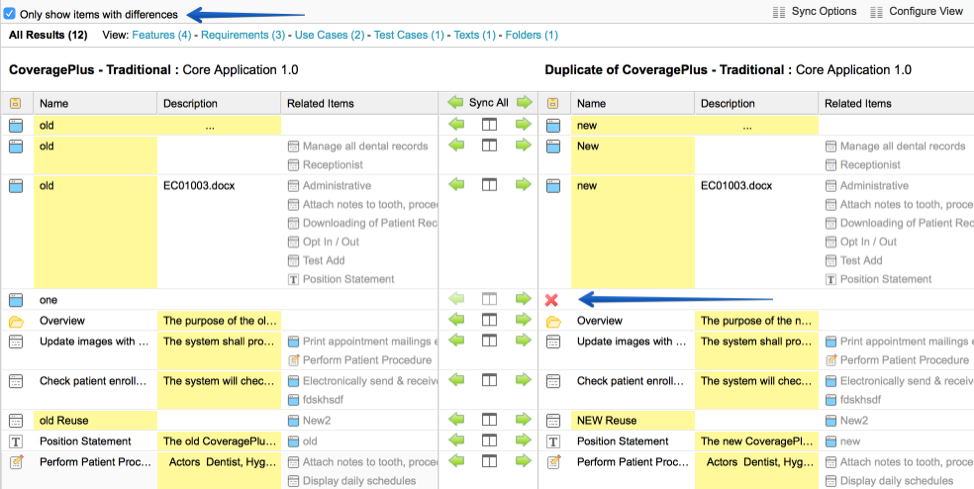
The Food and Drug Administration requires compliance with regulations such as FDA 21 CFR Part 11, regarding electronic records and e-signatures. Creating a detailed audit trail to comply with these rules is more straightforward if a unified system of record with full version histories – i.e., a software traceability solution – is in place. Jama Connect Review Center is compliant with FDA 21 CFR Part 11.
Modern traceability software maps out the relationships and interdependencies in product development, allowing for assiduous tracking of risks and requirements in their full historical context. Real-time collaboration also enables even geographically distributed teams to stay on the same page in tracking and tracing work items. This level of traceability, with visibility into who made each change and for what reasons, has become especially important as medical devices become more complex and software-driven.
In Jama Connect, risk analyses and other data such as product-specific views can also be easily exported to the correct formats to prove compliance. Industry-standard templates are available as well to minimize the setup time for creating a plan that aligns with standards such as ISO 14971:2019, ISO 13485, and FDA 21 CFR 820.30.
4. Connect Everyone and Everything with Trace Relationships
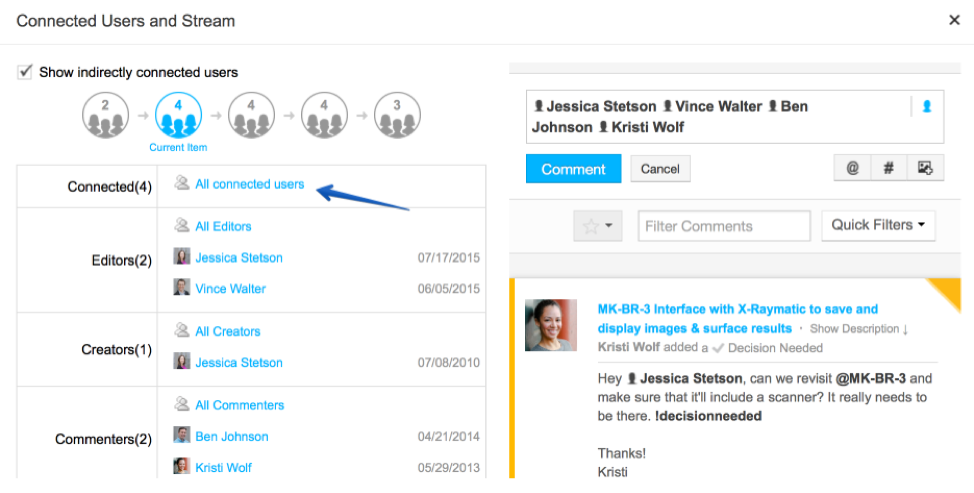
Traceability is about relationships. Because each product in development has its own particular set of customers, stakeholders, and internal team members associated with it, traceability is only possible if these individuals can be accurately connected to the items for which they are responsible.
That principle sounds simple enough on paper, but putting it into practice can be more complicated. Consider the question of who responds when a series of changes occurs and checks to see if everything is still right. With complex products, multiple team members will likely have to weigh in on and then sign off on these changes, a process that presents some big challenges:
- Decision-makers might not have clear visibility into the impact of changes to requirements, like their ripple effects on downstream or upstream items.
- High-quality data on requirements-related changes and why they were done might not be readily available and shareable, either.
- Coordinating the decision-making process itself can be cumbersome, with everyone exchanging documents via email and struggling to get in sync.
Fortunately, these obstacles can be overcome with traceability software. By implementing a shared system of record with real-time communications capabilities, organizations can adeptly manage their trace relationships and relieve the various forms of decision pressure outlined above.
5. Make Traceability More Proactive To Ensure Test Coverage
Traceability should not be pursued after the fact. Connecting requirements and other items at a relatively late stage is a recipe for trouble, as it can become difficult to gather all the necessary information and put into proper context. Extensive manual work, and the risks that come with it, are also likely to be required in such a reactive workflow.
In contrast, proactive traceability done throughout the product development lifecycle helps reduce risk and pave the way for higher-quality products that align with all of their requirements. Automation is key to this proactivity. Traceability software like Jama Connect automatically saves user inputs, can apply risk level updates in real time in accordance with a user-defined risk matrix, and ensures a standardized approach to risk evaluation across the organization – eliminating silos and disjointed processes.
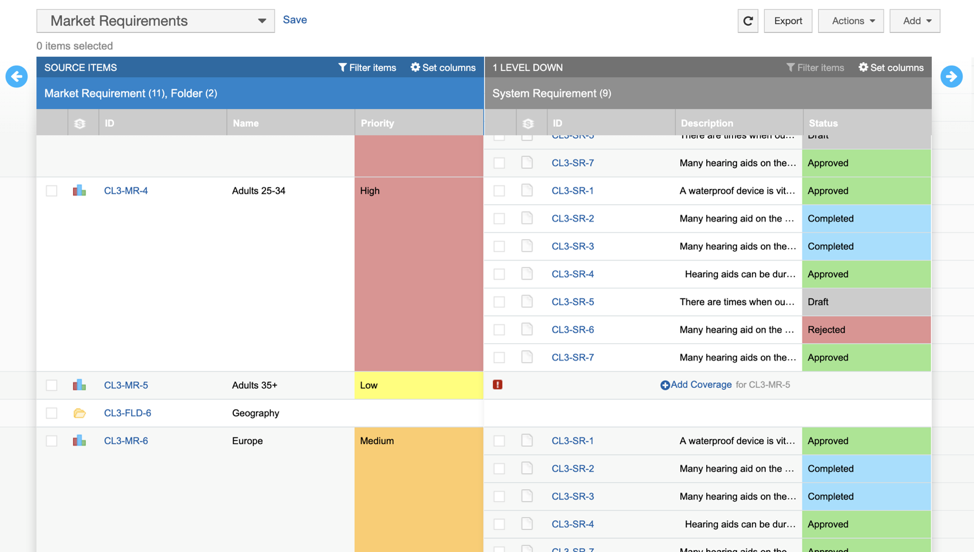
A traceability solution also provides clear visual representations of the status of requirements, test cases, and test runs along the way. These data-rich views enable better decision-making, since team members are working with live, easy-to-understand information and can communicate with each other through the same platform. For example, users can easily see test coverage levels and where gaps exist.
Take the Next Steps With Traceability Software
The right traceability software lets organizations efficiently manage all of your requirements in one place and streamline the development process of even the most complex products. It saves the entire team time and money, accelerates development lifecycles, reduces the risk of error, and results in improved product quality and regulatory compliance.
In short, traceability provides essential context and clarity. Look for a modern traceability solution that will keep projects on schedule, on budget, and within scope, thanks to better collaboration and requirements management.