Jama Connect’s Requirements Management Enables Live Traceability™ Across Your Development Process
Bridge engineering siloes across development, test, and risk activities. Provide end-to-end compliance, risk mitigation, and process improvement with our intuitive, award-winning requirements management platform. Learn more!
Audit trails are integral to regulatory compliance in industries ranging from medical device manufacturing to automotive production. But what’s the best way to build an audit trail that stands up to scrutiny?
Building a solid audit trail all starts with live traceability.
Why Live Traceability™ is Necessary
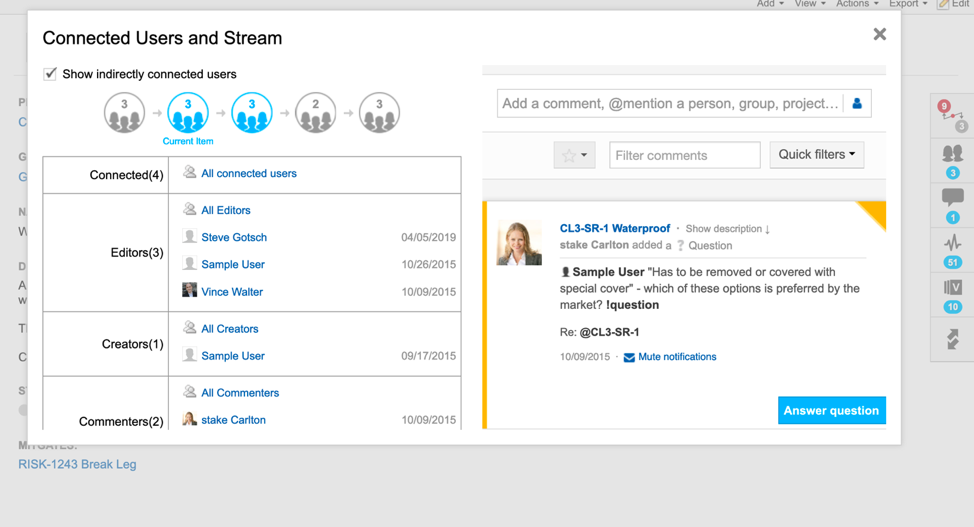
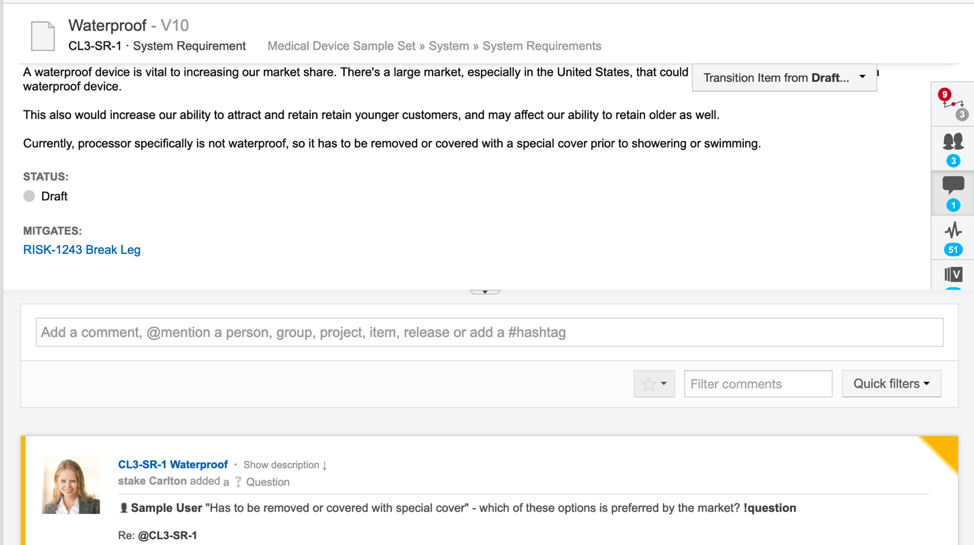
Live traceability is the tracking of requirements across the product development cycle. It documents the status of everything being worked on and shows the history of development along with the impacts of specific changes. Its benefits include easier regulatory compliance, more in-sync teams, and higher-quality releases.
In 2019, there were 50 medical devices recalled by the FDA — the most since 2014. Among other purposes, an audit trail helps strengthen product development controls and in turn curb the risk of recalls.
The top three reasons for recalls throughout the 2010s were device design flaws, software issues, and defective production processes. Audit trails document how such problems emerged. But too often, audit trails are assembled after the fact, without live traceability. Information may be pulled from numerous discrete documents, such as spreadsheets with hundreds of outdated entries. In contrast, live traceability happens along the way, adding supporting context for data relationships and contributing to better decision-making and future planning.
Live traceability makes it possible to both manage and respond to change in a systematic, auditable, and confidence-enhancing way. In our new infographic, we examine the ways that live traceability allows teams to build an audit trail.
RELATED POST: What is Requirements Traceability and Why Does it Matter for Product Teams?
Modern Product Development Requires Live Traceability™
One of the main reasons for implementing traceability software is to simplify regulatory compliance. Let’s say that a hypothetical medical device manufacturer is planning to bring a new connected device to the U.S. market.
The Food and Drug Administration requires compliance with regulations such as FDA 21 CFR Part 11, regarding electronic records and e-signatures. Creating a detailed audit trail to comply with these rules is more straightforward if a unified system of record with full version histories – i.e., a software traceability solution – is in place.
Modern traceability software (like Jama Connect) maps out the relationships and interdependencies in product development, allowing for assiduous tracking of risks and requirements in their full historical context. Real-time collaboration also enables even geographically distributed teams to stay on the same page in tracking and tracing work items. This level of traceability, with visibility into who made each change and for what reasons, has become especially important as products become more complex and software-driven.

 Editor’s Note: This post about moving beyond legacy systems for requirements management was originally published
Editor’s Note: This post about moving beyond legacy systems for requirements management was originally published