In the previous Jama Connect™ for Medical Device Development solution post, we learned how Jama Connect could:
- Establish and manage trace between design inputs and design outputs.
- Establish and manage trace between design inputs and verifications.
- Provide insight across these related Design Control activities.
Specifically, we saw how trace helped ensure alignment of these controls through acceptance criteria, increasing confidence in our verification activities and supporting visibility into the conformance of design outputs.
Capturing and managing design controls in Jama Connect expands the concept of traceability far beyond simply a reporting output required of the Design History File (DHF). Now, we’ll look at the importance of the trace in Jama Connect. It’s for more than connecting information for the sake of documentation. It’s a way of working. The matrix provided to the DHF becomes a byproduct of working in Jama Connect.
Use the trace to build efficiencies into the design and
development process.
Organizations that use documents and spreadsheets to manage requirements, verifications and risk management feel burdened with the overhead of trying to manage the trace of information across documents. I’ve worked with many teams who’ve said managing trace was the number one concern about their current way of working, and the reason for their transition to Jama Connect. When we talked about their desired state after deploying in Jama Connect, near or at the top of the list was no longer having to generate and manage the trace matrix.
Prior to Jama Connect, many of these organizations waited until the end of an effort to create their required matrix. While working with these customers, I realized the trace matrix should not be an effort in itself — it should be a byproduct of how teams work. When trace becomes just how teams work, not only do they remove the burden of having to generate it in the end, they can actually use the trace to build efficiencies into the design and development process.
The out-of-the-box configuration provided with the Jama Connect for Medical Device Development solution was constructed around the value of trace – not simply having trace but using trace.
Using the trace in the Jama Connect for Medical Device Development solution enables you to:
1. Manage scope through trace
Often, coverage, or gap, analysis is seen as a way of simply dotting i’s and crossing t’s to make sure a trace matrix shows full due diligence in product definition, verification and risk management. While very important, it serves only reporting what was done and as evidence after the fact. However, when this information is live and accessible, it becomes meaningful during design and development activities, not just at the end.
In the Jama Connect for Medical Device Development solution, teams can use trace views and filters to make assessments about work prioritization and managing scope. For example, the solution provides visibility into:
- Accepted system requirements lacking subsystem requirements to make sure they are working on the right things.
- Subsystem requirements not tied to a system requirement to understand and manage scope.
This way, gap analysis provides meaningful data in the moment for decision making and course correction.
2. Plan and identify connected activities
Often, team members authoring requirements, especially system requirements, have a sense of the lower level product definition and verification activities. However, they may not know the full picture or they may not be tasked with defining lower level definition.
Some examples:
- A systems engineer, in a top-down approach, may define a system requirement that will require interaction across software and electrical subsystems, though the engineer may not have the details.
- A hardware team, in a bottom-up approach, may identify a requirement of the firmware which needs to be managed through a system requirement.
In both examples, a higher-level design input, the system requirement, describes a function fulfilled across disciplines.
For Requirements
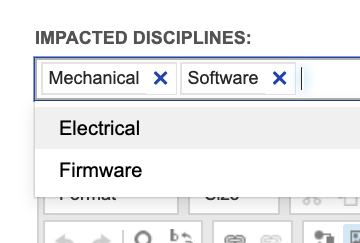
In Jama Connect we encourage capturing information about lower-level needs in the higher-level items, for allocation and for more detailed gap analysis. To handle the examples above, system requirements are configured to capture impacted disciplines, as shown below using a multi-select field:
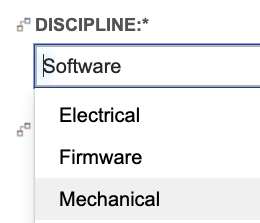
In addition to capturing the impacted disciplines in the system requirement, the configuration requires subsystem requirements capture the single owning discipline, as shown below using a single select field:
With this level of detail captured in system requirements and subsystem requirements, trace again proves useable throughout product definition activities. Filters provided with the out-of- the-box configuration give visibility into system requirements impacting specific disciplines but lacking a subsystem requirement for each impacted discipline. For example, we can easily identify system requirements allocated to software that lack a software requirement, same for any other disciplines identified.
This looks at system requirements from a gap analysis perspective (“where am I missing something?”). Another use of this same view identifies work to be done. Like the example above, if I am a Software Engineer, I can use that same filter to identify all accepted system requirements that I need to work on – those expecting but lacking a software response.
For Verification
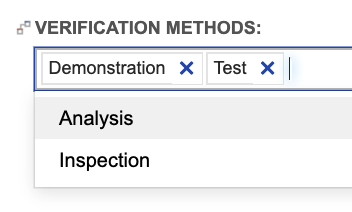
The concept described above for disciplines also applies to verification methods. The out-of-the box configuration of system and subsystem requirements provides a multi-select field for defining the verification method for the requirement:
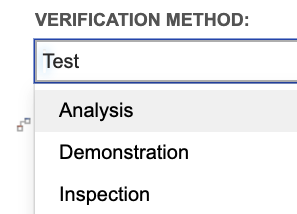
In the verification items, we capture the method in a single select field:
Just like we saw for requirement allocation to disciplines, we can use trace for more detailed gap analysis to begin defining verifications early, as requirements are accepted. For example, trace can tell us which requirements verified by “Testing” or “Inspection” lack a verification of that type. Testing teams can easily identify verification coverage gaps and address those gaps as requirements are accepted, instead of having to wait for large, fully formed requirement documents to be approved.
The benefits of trace.
The benefits of trace and the ability to use trace as described above are only possible if trace becomes a way of working. There are three keys to making this a reality:
1.Capture design controls in a single system.
Including design controls in a single system marks the fundamental requirement of a useful trace. It’s too much work to manage connections across multiple documents and there’s too little confidence in the manual maintenance of the trace to be useful. A single system, like Jama Connect, affords teams the opportunity to make trace useful and actionable.
2. Create through trace.
To encourage trace as a way of working, you have to make it easy to connect to new or existing information. You can create new information, both upstream and downstream, directly from related items In Jama Connect. This allows for authoring of new information and the automatic tracing of items.
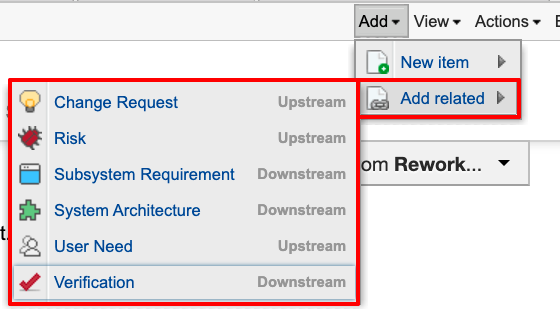
The image below shows just one way to establish the trace. This approach created new items directly from their related items.
We’re looking at a system requirement. I can create any of the listed related items directly from here, automatically establishing a trace. Note that what’s presented as options is determined by rules dictating what information can be traced. This ensures only valid traces are established. The Jama Connect for Medical Device Development solution enforces relationships through a relationship rule aligned to 21 CFR 820.30 and ISO 13485:2016.
3. Actively monitor trace
Jama Connect provides many ways to navigate item relationships, assess coverage and identify gaps. Trace View and Filters are important features supporting these activities. The key here is to act on the information these views provide. You can fill gaps and establish relationships established directly from these views. This is “actionable traceability.”
Catch up on all posts in the series.
We designed the Jama Connect for Medical Device Development solution to help device manufacturers realize the goal of designing and developing safe, effective and innovative Medical Device products. A goal we can all get behind!
Hopefully this series helps you as you work toward that goal. Each post had a purpose: to introduce the Jama Connect for Medical Device Development solution and describe components of the solution, to learn how to incorporate systems thinking into design inputs activities, then to understand design inputs, design outputs, verifications, and the relevant regulations and standards informing the solution, and finally, how to use the trace.
I hope it’s been informative and helpful. Through these concepts, the solution brings value and introduces visibility and efficiency into design control activities.
Stay up to date on the Jama Connect for Medical Device Development solution here.
- Jama Connect® Features in Five: Reuse & Sync - November 3, 2023
- Part V: Using the Trace as a Way to Work - June 25, 2020
- Part IV: Connecting Design Controls, Including Design Inputs, Design Outputs and Verifications - June 18, 2020