As medical device manufacturers develop complex products, they require a product development approach capable of managing that complexity.
At the same time, manufacturers must continue to ensure compliance and alignment with regulations and standards. These define requirements that ensure safety and quality and reduce risk—but ultimately do not prescribe specific tools or techniques.
This is especially apparent in design control activities. Regulatory requirements define the “what” for compliance but leave the “how” to the manufacturer, as long the procedures describing that “how” remain documented and prove sufficient as part of the quality system.
This lack of a prescribed approach to managing design controls can lead to uncertainty, but Jama Connect for Medical Device Development envisions that gap as an opportunity. Here, manufacturers have the space to deploy systems engineering techniques within design control activities, supported with a robust requirements, risk, and test solution.
Note: Now that our medical device blog series is concluded, you can go back and read the series intro, Part I, and Part II.
Bring systems engineering into the design control process to manage medical device complexity.
Systems Engineering solves the problem medical device manufacturers face. It takes a complex problem and divides it into manageable units so developers can see the solution both holistically and in its interrelated parts.
Aligning to 21 CFR 820.30 and ISO 13485:2016 can naturally guide manufacturers towards this approach, requiring trace across user needs, design inputs, design outputs and verifications. The guidance does not account for the abstraction required within these areas, especially design inputs, allowing the manufacturer to determine and implement appropriate techniques and tools.
The Jama Connect for Medical Device Development solution includes:
- A Procedure and Configuration Guide
- An out-of-the box configuration of Jama Connect
Together, these components bring Systems Engineering principles and design control requirements into a single recommended approach.
Here’s how.
1. Apply systems thinking.
The FDA guidance (FDA, 1997) indicates that product concepts are to be “elaborated, expanded, and transformed into a complete set of design input requirements.” Jama for Medical Device Development’s procedure guide applies systems engineering principles during this design input process.
- User needs are fulfilled by functions of the system.
- The system allocates them to specific engineering teams or product components for further, discipline-specific definition.
In some instances, like where software is the system, abstraction of design inputs from system-level to subsystems may not cross disciplines. However, the need for hierarchical product definition remains and is reinforced by software specific standards such as IEC 62304 and IEC 82304.
2. Capture and organize design inputs.
In Jama Connect, these levels of abstractions are managed as Item Types, discrete objects within Jama Connect that allow the solution to enforce rules for how information should trace to one another.
Concept-level information is captured as Intended Use and User Needs, engineering design responses as System and Subsystem Requirements. The total resulting set of requirements are referred to as the Design Inputs.
3. Establish the trace.
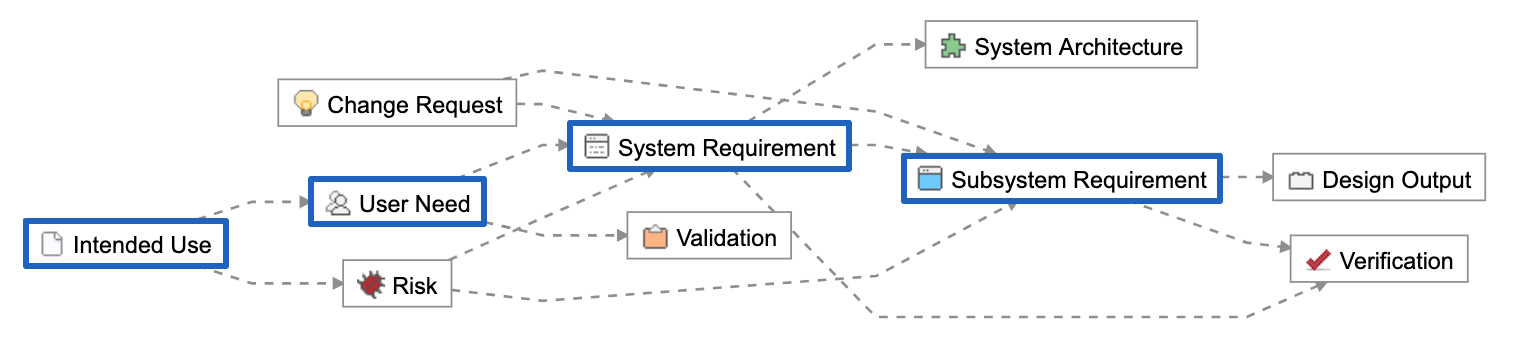
Below is a Relationship Rule provided as part of the out-of-the-box configuration of Jama Connect for Medical Device Development. In blue are the concept-level and design inputs:
These Item Types are unique data elements within Jama Connect. They allow us to create logical groupings we can use to manage the hierarchical levels of abstraction and to further organize across disciplines.
Standardizing engineering principles for requirements management using discrete Item Types is a valuable shift. It shows customers shifting focus from pure document generation to support for how they actually work, which can range from top-down, to middle-out, to bottom-up product definition.
The end result: Actionable information and accepted design inputs.
This shift to a focus on discrete items instead of whole documents encourages teams to act on information as it becomes ready. By capturing status within each individual item (e.g., a specific system requirement), items are independently driven from “Draft “to “Accepted.”
“Accepted” indicates a requirement is ready for:
- Documentation in the Quality Management System (QMS).
- Further decomposition or development.
- Defining Verification.
This single state can initiate several activities for other teams and does not require full document completion, which tends to restrict visibility and reduce momentum.
Although this approach encourages a shift from a document-focused product definition, in reality a document needs to be generated for the Design History File (DHF). To support these potentially conflicting needs, the out-of-the-box configuration:
- Drives organization of information based on a systems engineering approach.
- Uses Jama Connect’s filtering capabilities to pull together information across the project for document generation.
Using filters for document generation allows you to do more than collect different types of requirements for a single document. You can use data within the items, specifically in the Status of the items, to generate a document of only accepted design inputs. You can also take a baseline as part of the document generation process. The result is a snapshot of accepted design inputs that you can use for comparison reporting to show how design inputs may have changed over time. You can also indicate in each item’s version history which version of a requirement was included in the generated documents.
Use this Design Inputs approach with the Jama Connect for Medical Device solution to make it easier to generate documentation you need while supporting how you work with systems engineering approaches.
In the next post in this series, I’ll show you how to connect design inputs with design outputs and verifications.
Watch a demo to see key Jama Connect Medical Device Development Solution features in action and understand how teams use it to support their development process.
- Jama Connect® Features in Five: Reuse & Sync - November 3, 2023
- Part V: Using the Trace as a Way to Work - June 25, 2020
- Part IV: Connecting Design Controls, Including Design Inputs, Design Outputs and Verifications - June 18, 2020