In this blog, we recap our whitepaper, “Applications of Systems Engineering in Healthcare” – Download the complete paper HERE.
Applications of Systems Engineering in Healthcare
When it comes to healthcare, time to market is one of the most crucial aspects of success or failure. However, medical product development teams face several challenges that slow product development, and in the quest to speed up the process, some teams are turning to systems engineering to improve the process.
In this whitepaper, we’ll look at the challenges healthcare development teams face, the difference between market-driven and contract-driven industries, and how the power of simplicity can help healthcare systems engineering teams strike a perfect balance to adapt, innovate, and succeed.
The Challenges of Healthcare Systems Development
To understand how systems engineering can help, it’s important to first look at the challenges development teams face.
First, teams must balance time demands with the need to launch products that are both safe and effective. Today, the time to define requirements has increased by 29%, and unplanned requirements churn has increased by 81%, resulting in about 70% of medical products being delivered late.
The shifting regulatory landscape presents more challenges, including the increased cost of adherence to such regulations as Software as a Medical Device (SaMD), Software in a Medical Device (SiMD), Medical Device Regulation (MDR), and In Vitro Diagnostic Regulation (IVDR). At one of the top medical device development firms, for example, their product developers had to monitor approximately 8,000 regulations. Ensuring that products meet quality, safety, and performance standards has a significant financial impact; getting it wrong can cost billions of dollars. Across the industry, non-routine quality events cost between $2.5 and $5 billion per year.
In addition to increasing design complexity, there is also an increase in process complexity. Software development teams have gone from between 20 and 40 people to hundreds of people. Artificial intelligence (AI), machine learning (ML), and other new technologies represent complexity inside devices. Organizations are getting more complex as well, with a heavy focus on acquisition, which means constantly integrating new teams and cultures, sometimes dispersed across the globe.
Systems engineering can help product developers in healthcare manage these complexities and streamline development to keep them competitive in a rapidly changing market.
RELATED: The Complete Guide to the Systems Engineering Body of Knowledge (SEBoK)
Market-Driven vs. Contract-Driven
To understand how systems engineering can improve speed to market, it’s important to first understand the difference between a “market-driven” and a “contract-driven” industry.
In a market-driven industry, the first mover tends to get the lion’s share of the profits. Market-driven industries have many customers, and the stakeholders are internal to the business. Budget, time, and requirements are negotiated within the organization.
In a contract-driven industry, success means satisfying the contract. Budget and time are fixed by the contract with one (or very few) customers. In this scenario, requirements are a key commitment negotiated within formal design control.
The two different industry models present very different requirements challenges. In a market-driven industry, requirements are an internal business tool that helps communicate across business functions. They must be validated, but the development team decides on timing and features. If a team member develops a new, innovative feature, everyone can agree to take extra time to develop it. In a contract-driven industry, that likely wouldn’t be possible given the constraints of the contract.
Systems engineering can help the market-driven industry turn ambiguous needs into clear and feasible solutions to be implemented by hardware and software teams.
Systems Engineering: From Needs to Solutions
Product developers in a market-driven industry receive a lot of input from the various stakeholders within the organization. Their task is to turn that input into marketable products that work seamlessly on day one, day fifty, and years later. The key value produced is the seamless integration of those products into every customer’s workflow and work systems. Every installation and every service event must produce a uniform, high-quality, high-performing product.
Within those constraints, developers need to optimize the business value. When there are multiple options, marketing will inform the team of the customer value of these options. The implementation teams will pass on the delivery and product costs of those functions. The role of systems engineering is to make trade-offs between those and optimize the business impact based on the cost of implementing them. Associated with that is managing technical risks and scaling costs by risk.
The key value of systems engineering is making sure design decisions are identified and closed predictably with one voice across the team. Decisions are framed, the options are agreed to, the decision criteria are agreed to, and the final decision is closed, and stays closed even as stakeholders change. Once the team has a frozen design, integration or quality problems can be found and resolved prior to moving on to the next phase. By creating time to react, teams allow themselves space to adjust design early in the program rather than rushing to fix quality issues before shipping.
Winning products happen when systems thinkers are effective. When everyone across the program engages in systems thinking, the team will maximize the creativity of the entire program.
RELATED: How to Overcome Three of the Biggest Challenges in Medical Device Development
What is Systems Engineering in Healthcare?
As a process example, at one leading US-based medical device development company, engineering teams start with the end customer’s performance requirements, such as delivering excellent image quality in their imaging
products or the proper humidity and temperature for neonatal products. As part of delivering that essential performance, teams must ensure safety and regulatory compliance.
Their product teams also put a high emphasis on usability, ensuring that their products are easy to use and delight the customer. The teams define the right implementation requirements and reliability strategy, and they ensure that their products can be installed and serviced properly.
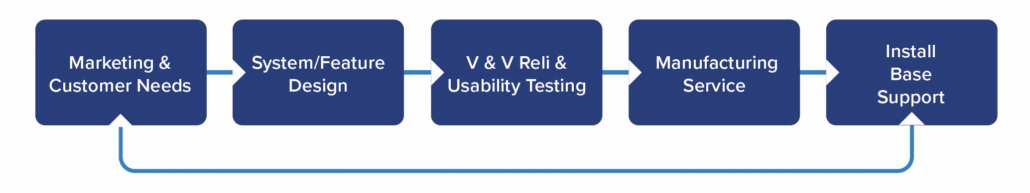
While there is tremendous diversity in products and programs across most medical device and life sciences companies, there are several commonalities across the product teams as well. Teams have common program milestones and a common systems’ lifecycle based on the V-model with iteration and Agile built in.
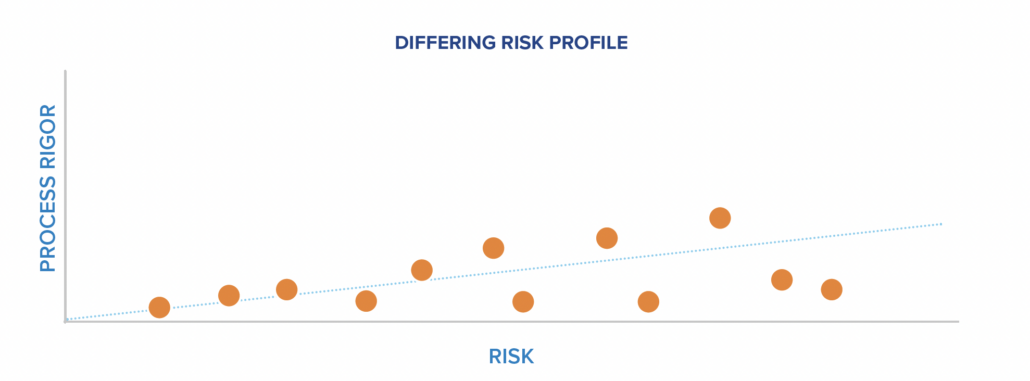
What differs in product teams are the levels of safety hazards and FDA risk. Teams develop everything from anesthesia technology, which could easily kill a patient, to ultrasound, which is non-ionizing equipment operated with light, handheld probes. To accommodate these different levels of risk, teams adjust the process rigor so that higher-risk modalities have higher process rigor.
Additionally, systems engineering teams can look very different across the world. Many organizations operate in different locations with different cultures and different organizational sizes. Systems engineering teams can vary from fewer than ten engineers to over one hundred engineers. The scale of the programs can range from just a few engineers over a few months to many hundreds of engineers applied to a program that might last three years and is based on technology developed over the prior decade. (Even in that research phase, teams should apply some systems engineering thinking.) Organizations can be product-centralized or decentralized within an organization.
TO LEARN MORE, DOWNLOAD THE COMPLETE WHITEPAPER HERE:
“Applications of Systems Engineering in Healthcare”
- [Webinar Recap] Application of Systems Engineering in Healthcare - August 29, 2024
- Applications of Systems Engineering in Healthcare - March 28, 2024