Medical Device Risk Management
Medical device developers must ensure risk is addressed as a core activity. The ISO 14971 standard, which has been revised three times, provides a proven and flexible framework around which developers can effectively manage the risk of devices for patients and stakeholders. Knowing the standard and applying some aspects, in a reactive way, is not sufficient to create a safe product. Implementing an effectively proactive process and achieving live traceability throughout development, while adhering to the standard will result in a higher quality and safer product fit for market use.
In this blog, I will share how proactive risk management along with Live Traceability™ are two key areas that every medical device developer should focus on when it comes to risk management.
Reactive vs. Proactive
A lot of medical device developers do not prioritize risk evaluation throughout the different design stages, but instead, consider risk a checkbox activity at the end of development. With important prototype deadlines, limited funding, and resource constraints, it’s very easy to make excuses for not running risk evaluations of initial user needs but waiting until entire subsystems have been designed. This may seem like a minor hiccup, but the lack of ongoing risk assessment from an early stage can be very risky for FDA approval, future patients as well as your business’s bottom line and reputation. The later that risk is addressed, the more expensive changes are to incorporate. Delays become the norm as well as budget overruns.
Live Traceability™
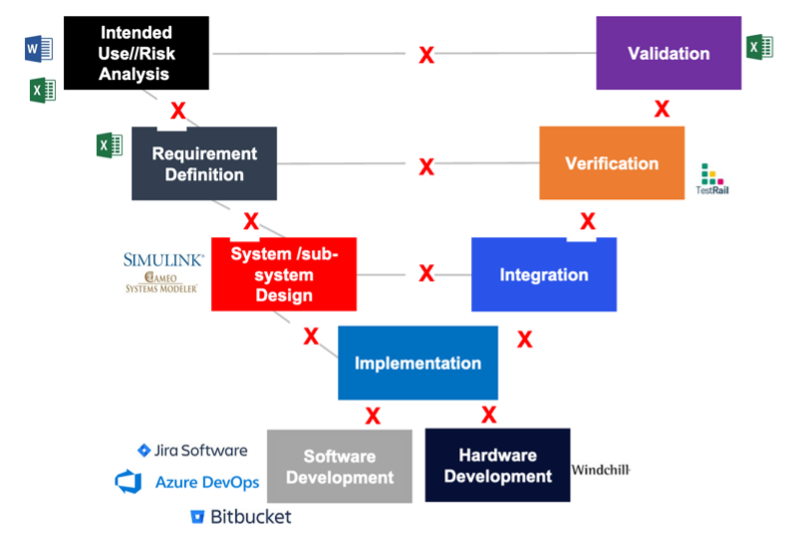
The number one cause of inspectional observations, product recalls & delays, increased CAPAs, and cost overruns is the lack of live traceability throughout different development stages. With complex medical devices, there can be thousands of user needs, product requirements, risks, mitigating requirements, tests being executed, and defects logged. At Jama Software, we’ve noticed that a lot of medical device developers are using different technologies and applications to store this information including Excel, Jira, Word, and others. The below image depicts that and shows the siloed world a lot of engineering teams are living in:
Unless you have a real-time system that can interface with spreadsheets, tools like Jira and other applications, it’s impossible to get a holistic view of development. This can cause several issues, some of the most critical being:
- Late identification of defects/coverage gaps due to lack of visibility throughout the development process
- Lack of requirement coordination and change management between hardware/software
- Lack of ongoing risk assessment and change management
- Cumbersome and risky effort to produce trace reports and other DHF artifacts
RELATED POST: Avoid the Most Common Challenges of the Design History File
Without Live Traceability, risk teams can’t be effective in mitigating potential failures or hazards. Outdated and manually updated trace reports and spreadsheets can’t be relied upon. What if there was a way to get a real-time view of traceability throughout development?
At Jama Software, we’ve created the capability for our product development platform to integrate seamlessly with tools like Excel, Jira, and others straight out of the box, and we are proud to be the only vendor on the market that can help engineering teams realize Live Traceability. Risk should be a priority, not a checkbox item!
Get in touch with us to see how we can help you.
- [Webinar Recap] Achieving Success in Energy Storage Development: Tips & Best Practices - September 10, 2024
- [Webinar Recap] Managing Functional Safety in Development Efforts for Robotics Development - August 6, 2024
- [Webinar Recap] Excelling in Requirements Management for Successful Software Delivery and Implementation - August 1, 2024