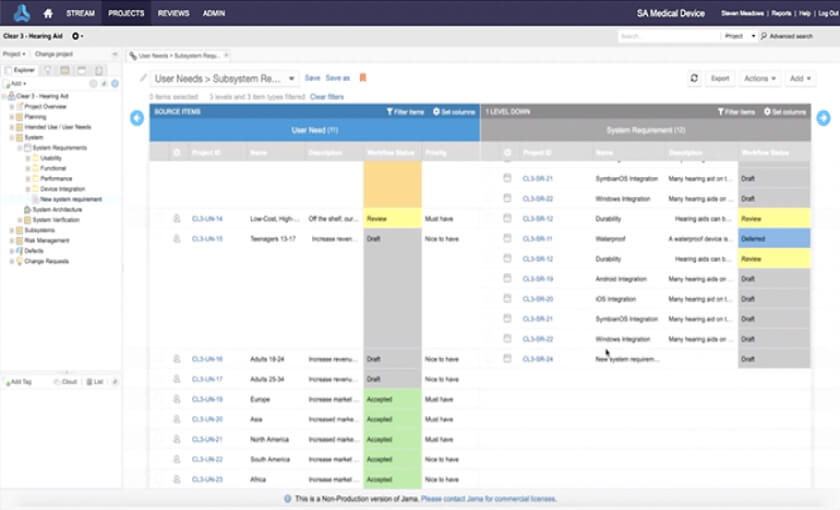
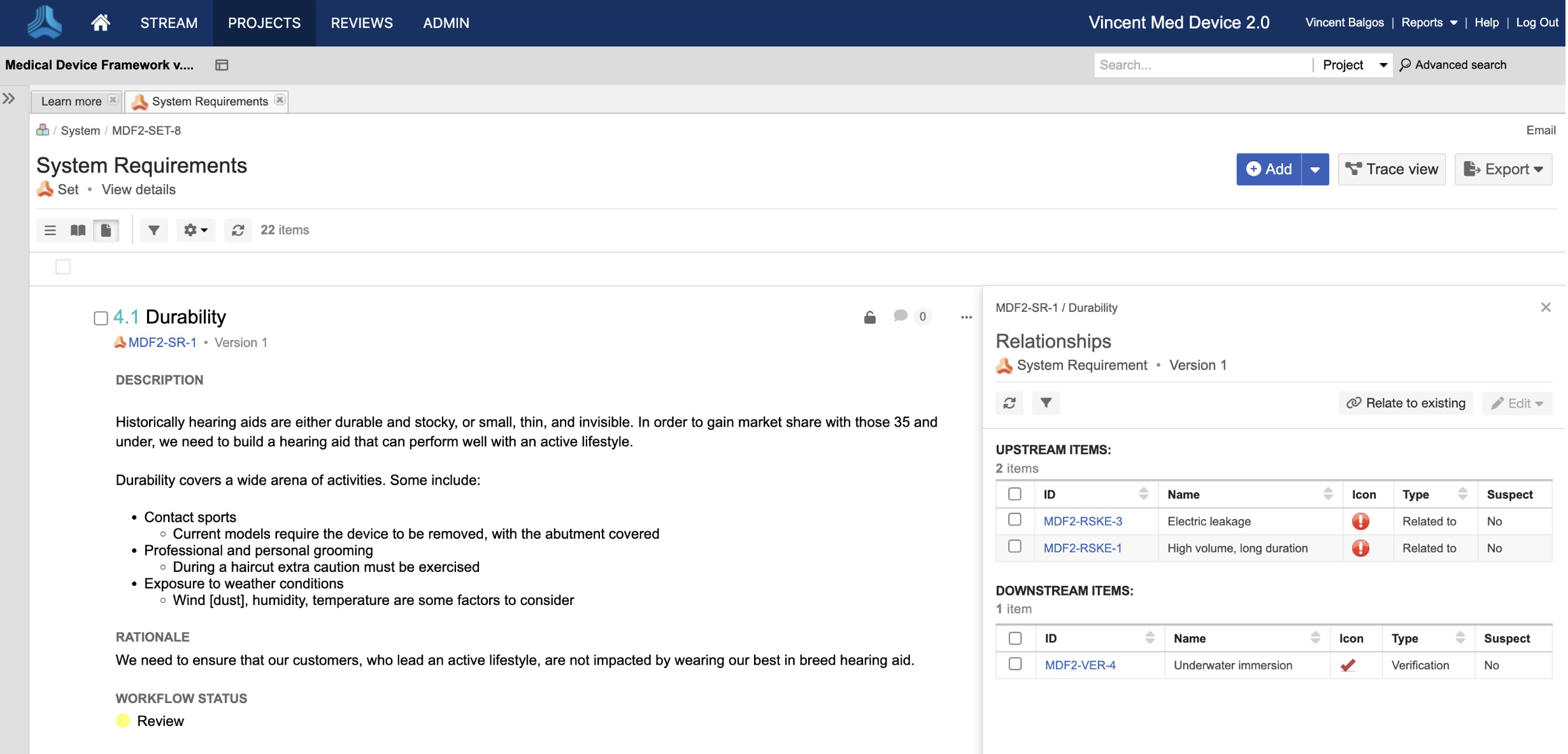
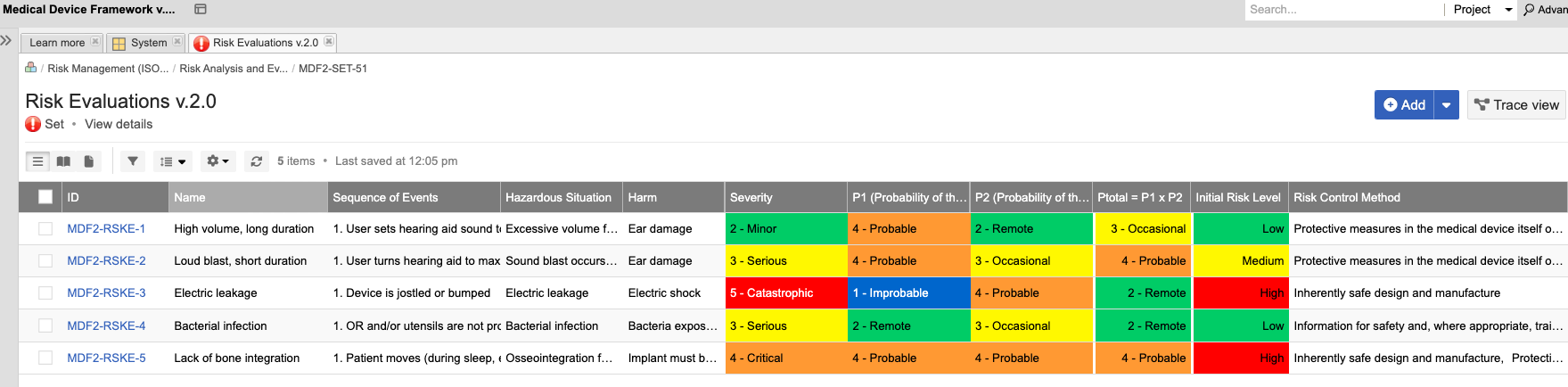
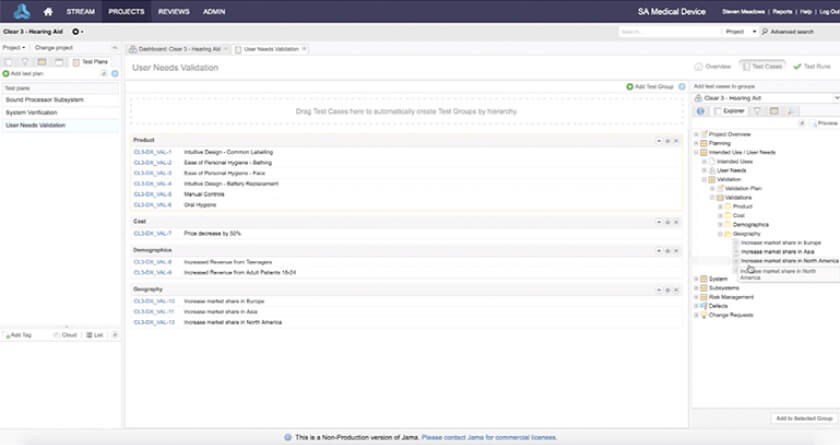
Accelerate the Development of Safe and Effective Medical Devices & Life Science Products with Jama Connect
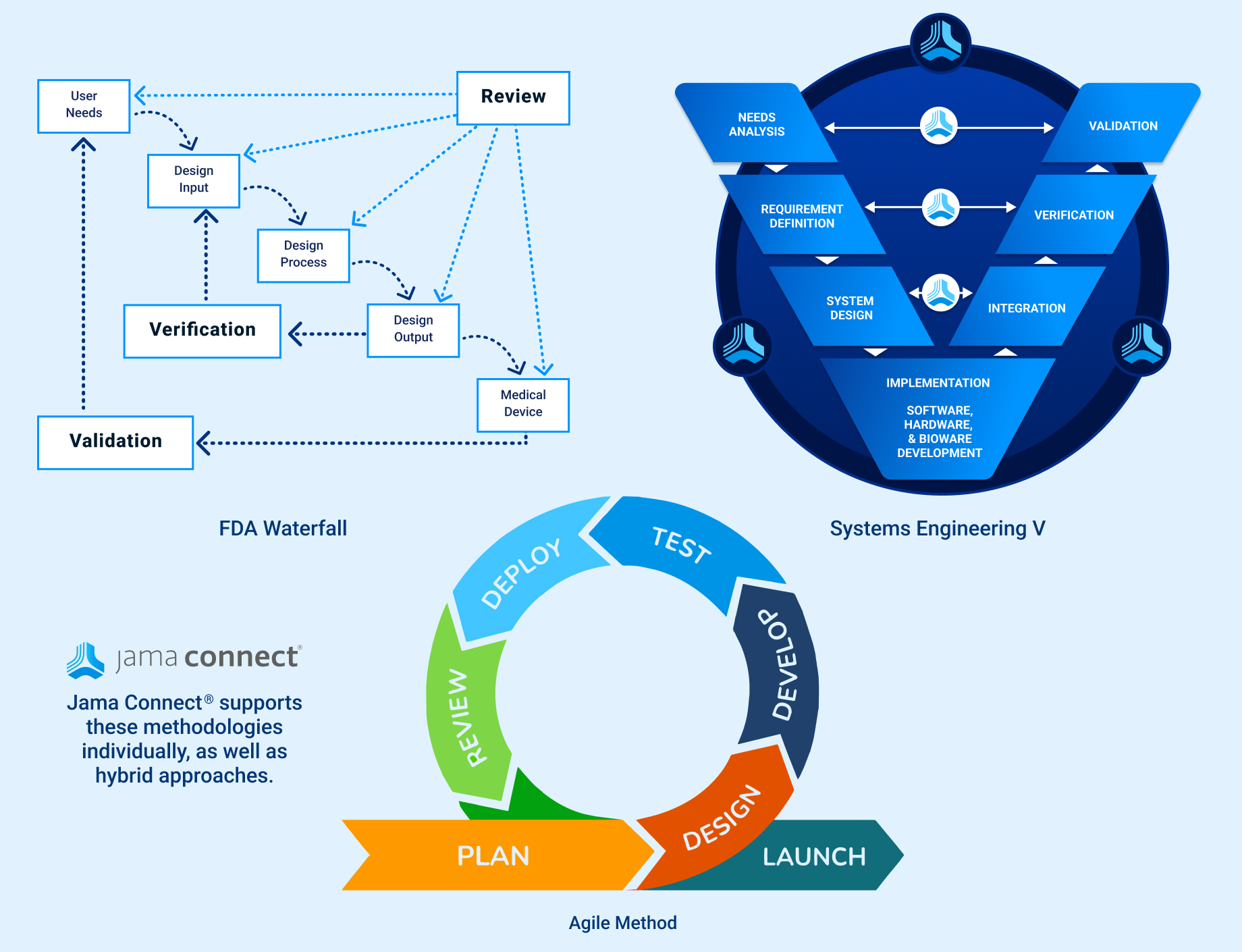
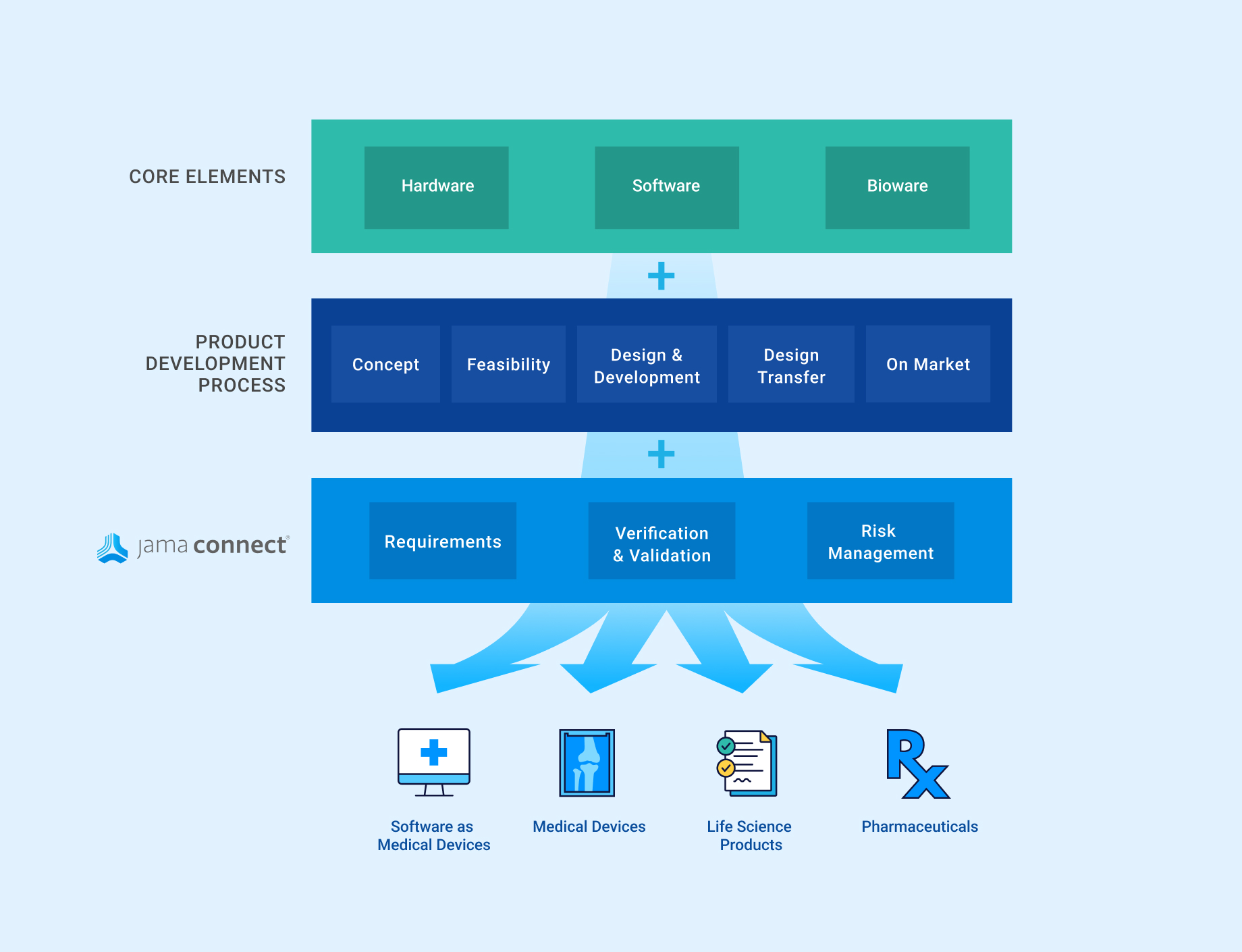
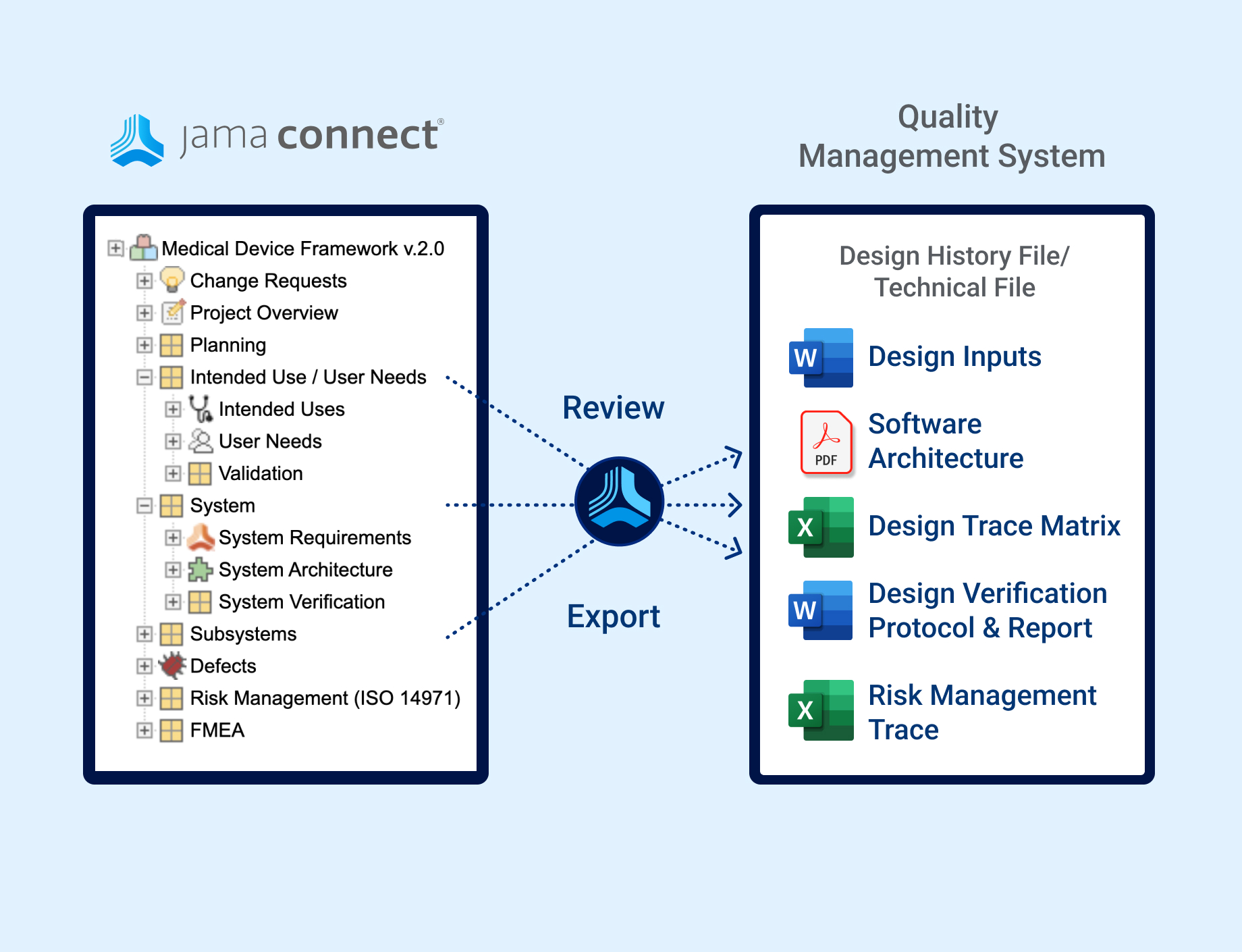
In the medical device & life sciences industries, the transformation from hardware-focused devices to diverse products driven by software — in combination with hardware and bioware — is reshaping the tools and processes by which devices and related products are developed. Jama Connect for medical device requirements management is purpose-built to reduce the effort required to achieve regulatory compliance by managing design controls for device requirements and related risks, simplifying regulatory submissions and audit preparations, and accelerating time to market.