In the previous blog of this series, we talked about the application of systems engineering principles to the design inputs process. In this post, we explore how the Jama Connect™ for Medical Device Development procedure guide describes connecting design inputs with subsequent processes: Design Outputs and Verifications. By supporting these processes in a single system and ensuring traceability we can build confidence in the conformance to design inputs and the proper definition of verification activities.
The Jama Connect for Medical Device Development solution focuses on two key aspects from the Design Output requirements defined by 21 CFR 820.30 and ISO 13485:2016 section 7.3.4. Design outputs:
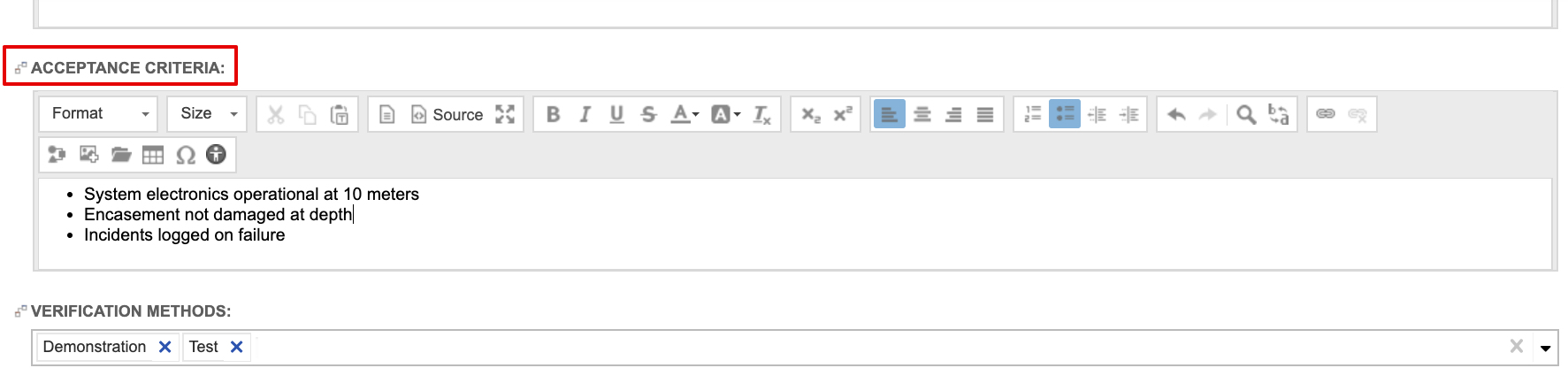
- must reference acceptance criteria, ensuring they are essential to proper functioning,
- are evaluated for conformance to design input requirements
Design outputs will vary based on the product, technology, discipline and applicable standards.
The regulation and standards are not specific about the content of the design outputs themselves. The significant activity then from a design control perspective is ensuring a trace to design inputs and visibility into the verification of those design inputs.
When defining design inputs (i.e., system and subsystem requirements), the guide recommends stating acceptance criteria directly in the requirement items. In fact, the system and subsystem requirements are configured capture it out of the box. Below is a sample System Requirement using the out of the box system requirement configuration.
Verification items, traced to design inputs, should be defined based on the acceptance criteria. Similarly, design output items (e.g., architecture diagrams) are traced to design inputs and refer to requirement details and acceptance criteria for design and development.
For some disciplines, like Software, Jama Connect may contain more detailed levels of abstraction and, therefore, may have items that tie directly into development tools. For other disciplines, Jama Connect may contain very basic information and pointers to other systems managing design outputs details, like parts for manufacturing.
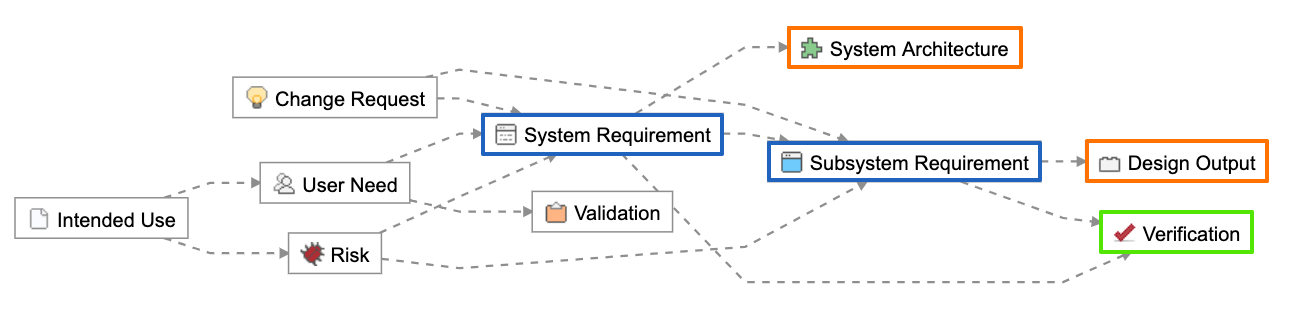
The Relationship Rule below provided with the Jama Connect for Medical Device Development out of the box configuration shows how design outputs (in orange) and verifications (in green) trace directly to design inputs (in blue).
Using this approach, the design input serves as the point connecting design outputs and verifications. Design outputs contain or point to evidence of the implementation of the design input, while verifications provide evidence through testing that the intent of the design input is met. Both design outputs and verifications reference the acceptance criteria captured in the design input during their definition and by nature of the trace.
Connecting design inputs with design outputs and verifications.
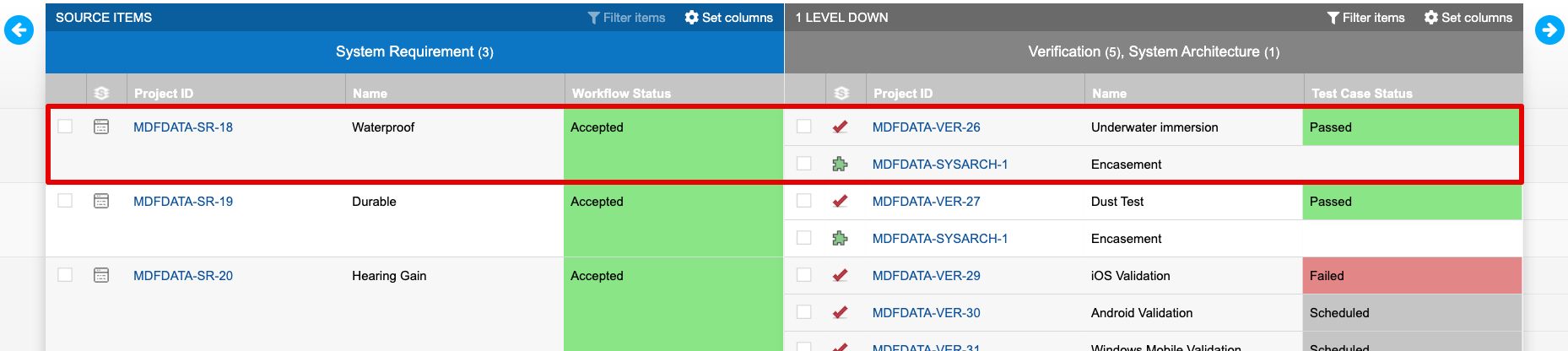
The trace established by connecting design inputs with design outputs and verifications is visible in Jama Connect’s Trace View. The sample of a Trace View below shows a requirement with two related items downstream, a verification and design output. The verification in this example shows “Passed”, which indicates the intent of the requirement was met via implementation of the related design output.
By capturing acceptance criteria during the Design Input process, exposing that information to related downstream processes, and using trace as a part of normal product definition activities, manufacturers gain visibility into the conformity of design outputs and build confidence in their verification activities and results. As seen in the Trace View above, relating information in Jama Connect is key to understanding coverage and verification.
In the next blog in this series, we’ll explore making further use of the trace, beyond simply matrix generation.
Haven’t read the earlier blogs in the series? You can catch up here.
Part One: Jama Connect™ for Medical Device Development Explained
Part II: Solution Components of Jama Connect for Medical Device Development
Part III: Design Inputs in Jama Connect for Medical Device Development
Watch a demo to see Jama Connect Medical Device Development Solution features in action and understand how teams use it to support their development process.
- Jama Connect® Features in Five: Reuse & Sync - November 3, 2023
- Part V: Using the Trace as a Way to Work - June 25, 2020
- Part IV: Connecting Design Controls, Including Design Inputs, Design Outputs and Verifications - June 18, 2020